Small cationic antimicrobial peptides delocalize peripheral membrane proteins - PubMed (original) (raw)
. 2014 Apr 8;111(14):E1409-18.
doi: 10.1073/pnas.1319900111. Epub 2014 Mar 24.
Alina Iulia Chiriac, Andreas Otto, Dagmar Zweytick, Caroline May, Catherine Schumacher, Ronald Gust, H Bauke Albada, Maya Penkova, Ute Krämer, Ralf Erdmann, Nils Metzler-Nolte, Suzana K Straus, Erhard Bremer, Dörte Becher, Heike Brötz-Oesterhelt, Hans-Georg Sahl, Julia Elisabeth Bandow
Affiliations
- PMID: 24706874
- PMCID: PMC3986158
- DOI: 10.1073/pnas.1319900111
Small cationic antimicrobial peptides delocalize peripheral membrane proteins
Michaela Wenzel et al. Proc Natl Acad Sci U S A. 2014.
Abstract
Short antimicrobial peptides rich in arginine (R) and tryptophan (W) interact with membranes. To learn how this interaction leads to bacterial death, we characterized the effects of the minimal pharmacophore RWRWRW-NH2. A ruthenium-substituted derivative of this peptide localized to the membrane in vivo, and the peptide also integrated readily into mixed phospholipid bilayers that resemble Gram-positive membranes. Proteome and Western blot analyses showed that integration of the peptide caused delocalization of peripheral membrane proteins essential for respiration and cell-wall biosynthesis, limiting cellular energy and undermining cell-wall integrity. This delocalization phenomenon also was observed with the cyclic peptide gramicidin S, indicating the generality of the mechanism. Exogenous glutamate increases tolerance to the peptide, indicating that osmotic destabilization also contributes to antibacterial efficacy. Bacillus subtilis responds to peptide stress by releasing osmoprotective amino acids, in part via mechanosensitive channels. This response is triggered by membrane-targeting bacteriolytic peptides of different structural classes as well as by hypoosmotic conditions.
Keywords: hypoosmotic stress response; mechanism of action; metallocenes; respiratory chain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
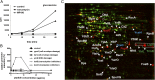
Narrowing down the target area. (A) [3H]-glucosamine incorporation by S. simulans upon treatment with MP196 or vancomycin. (B) Transcription activation of selected B. subtilis promoters indicative of inhibition of specific cellular processes. Cells were incubated with increasing concentrations of MP196 for predetermined times depending on the induction kinetics of the respective reporter strains. (C) 2D gel-based proteome analysis of MP196-treated B. subtilis. Synthesis rates of cytosolic proteins of MP196-treated (false-colored in red) and untreated (false-colored in green) B. subtilis were compared based on [35S]-methionine labeling. In the overlaid autoradiographs, down-regulated proteins appear green, up-regulated proteins appear red, and proteins expressed at equal rates appear yellow. Unidentified proteins are marked by circles. Blue labels indicate marker proteins for general cell-envelope stress, green labels identify cell-wall biosynthesis inhibition, and red labels are markers for membrane stress.
Fig. 2.
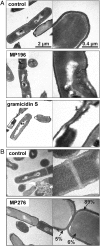
Peptide effects on cells and subcellular localization. (A) TEM images of peptide-treated B. subtilis. Cells were fixed with 2% uranyl acetate, and ultrathin sections were stained with 0.2% lead citrate in 0.1 M NaOH. (B) In vivo peptide tracing using the ruthenocene-substituted MP196 derivative MP276. B. subtilis cells were fixed with 2% uranyl acetate. Sections remained unstained to avoid interference of lead with the ruthenium signal. Ruthenium in the cytosol, membrane, and cell wall was quantified by atomic absorption spectroscopy.
Fig. 3.
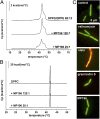
Interaction with the membrane. (A) DSC thermograms of lipid bilayers consisting of 88:12 DPPG/DPPE incubated with MP196. Changes in thermotropic-phase behavior caused by perturbation of fatty acyl packing indicate peptide integration. (B) DSC thermograms of lipid bilayers consisting of DPPC. (C) Overlaid fluorescence microscopy images of _Bac_Light-stained B. subtilis treated with valinomycin, nisin, gramicidin S, and MP196. A red-fluorescing dye selectively stains cells with large membrane holes; a green-fluorescing dye stains all cells independently of membrane integrity. Cells with intact membranes appear green, and cells with perforated membranes appear orange.
Fig. 4.
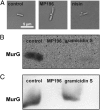
Effects on membrane potential and respiration. (A) Fluorescence microscopy images (Upper Row) and corresponding bright-field images (Lower Row) show GFP-MinD localization in untreated B. subtilis cells and cells incubated with gramicidin S or MP196. GFP-MinD delocalization indicates membrane depolarization. (B) ATP levels of B. subtilis cells treated with valinomycin, nisin, gramicidin S, or MP196. In a luciferase assay, ATP concentrations were determined under conditions resembling those of the proteome experiment. (C) Activity of the respiratory chain of inverted M. flavus vesicles treated with rotenone, antimycin A, or MP196. Electron transport efficiency was monitored by the reduction of iodonitrotetrazolium chloride causing a decrease in absorbance at 485 nm. (D) Western analysis of cytochrome c in membrane fractions of B. subtilis after a 5-min treatment with gramicidin S and MP196. The Ponceau S-stained blot is displayed as loading control. (E) Overview of the bacterial respiratory chain [according to Vonck and Schäfer (38)]. Proton movements are indicated by green arrows; electron movements are indicated by orange arrows.
Fig. 5.
Effects on the cell wall. (A) Light microscopy images showing cell-wall integrity of B. subtilis after treatment with MP196 and nisin. Acetic acid-methanol fixation visualizes cell-wall damage by extrusions of the membrane through holes in the cell wall. (B) Western blot detection of the cell-wall biosynthesis protein MurG in membrane fractions isolated from B. subtilis cells that were treated with peptide for 5 min. (C) Western blot detection of MurG in B. subtilis membrane fractions that first were isolated and then were incubated with peptide for 5 min.
Fig. 6.
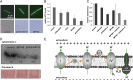
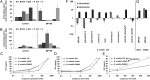
Amino acid composition. (A) HPLC analysis of the intracellular amino acid composition of B. subtilis treated with MP196. (B) Extracellular amino acid composition of the same cultures. Only the amino acids whose concentrations changed significantly after peptide treatment are displayed here (see
SI Appendix, Fig. S8
for full amino acid profiles). Amino acids are written in a one-letter code in the order of elution time from the column. Glutamate and glutamine as well as aspartate and asparagine are not distinguishable by this method and appear as one peak each. Tryptophan was not quantified here. (C_–_E) MICs of MP196 against B. subtilis 168, a B. subtilis strain lacking all known mechanosensitive channels (SMB80), and its parent strain (JH642) in defined medium supplemented with increasing glutamate (C), NaCl (D), or KCl (E) concentrations. MICs were determined independently twice. (F) Intra- and extracellular glutamate concentrations in B. subtilis cells under different antibiotic and osmotic stress conditions determined by amino acid analysis. (G) Intra- and extracellular glutamate concentrations in a B. subtilis JH642 and SMB80.
Similar articles
- Influence of lipidation on the mode of action of a small RW-rich antimicrobial peptide.
Wenzel M, Schriek P, Prochnow P, Albada HB, Metzler-Nolte N, Bandow JE. Wenzel M, et al. Biochim Biophys Acta. 2016 May;1858(5):1004-11. doi: 10.1016/j.bbamem.2015.11.009. Epub 2015 Nov 18. Biochim Biophys Acta. 2016. PMID: 26603779 - Synthetic antimicrobial peptides delocalize membrane bound proteins thereby inducing a cell envelope stress response.
Omardien S, Drijfhout JW, van Veen H, Schachtschabel S, Riool M, Hamoen LW, Brul S, Zaat SAJ. Omardien S, et al. Biochim Biophys Acta Biomembr. 2018 Nov;1860(11):2416-2427. doi: 10.1016/j.bbamem.2018.06.005. Epub 2018 Jun 9. Biochim Biophys Acta Biomembr. 2018. PMID: 29894683 - Antimicrobial activity of arginine- and tryptophan-rich hexapeptides: the effects of aromatic clusters, D-amino acid substitution and cyclization.
Wessolowski A, Bienert M, Dathe M. Wessolowski A, et al. J Pept Res. 2004 Oct;64(4):159-69. doi: 10.1111/j.1399-3011.2004.00182.x. J Pept Res. 2004. PMID: 15357671 - The interaction of antimicrobial peptides with membranes.
Travkova OG, Moehwald H, Brezesinski G. Travkova OG, et al. Adv Colloid Interface Sci. 2017 Sep;247:521-532. doi: 10.1016/j.cis.2017.06.001. Epub 2017 Jun 5. Adv Colloid Interface Sci. 2017. PMID: 28606715 Review. - Mechanical properties that influence antimicrobial peptide activity in lipid membranes.
Marín-Medina N, Ramírez DA, Trier S, Leidy C. Marín-Medina N, et al. Appl Microbiol Biotechnol. 2016 Dec;100(24):10251-10263. doi: 10.1007/s00253-016-7975-9. Epub 2016 Nov 11. Appl Microbiol Biotechnol. 2016. PMID: 27837316 Review.
Cited by
- Mechanisms of action of ionic liquids on living cells: the state of the art.
Kumari P, Pillai VVS, Benedetto A. Kumari P, et al. Biophys Rev. 2020 Oct;12(5):1187-1215. doi: 10.1007/s12551-020-00754-w. Epub 2020 Sep 16. Biophys Rev. 2020. PMID: 32936423 Free PMC article. Review. - An Enhanced Variant Designed From DLP4 Cationic Peptide Against Staphylococcus aureus CVCC 546.
Li B, Yang N, Wang X, Hao Y, Mao R, Li Z, Wang Z, Teng D, Wang J. Li B, et al. Front Microbiol. 2020 Jun 5;11:1057. doi: 10.3389/fmicb.2020.01057. eCollection 2020. Front Microbiol. 2020. PMID: 32582062 Free PMC article. - Cysteine Potentiates Bactericidal Antibiotics Activity Against Gram-Negative Bacterial Persisters.
Liu Y, Yang K, Jia Y, Shi J, Tong Z, Wang Z. Liu Y, et al. Infect Drug Resist. 2020 Jul 28;13:2593-2599. doi: 10.2147/IDR.S263225. eCollection 2020. Infect Drug Resist. 2020. PMID: 32801796 Free PMC article. - Therapeutic Potential of Gramicidin S in the Treatment of Root Canal Infections.
Berditsch M, Lux H, Babii O, Afonin S, Ulrich AS. Berditsch M, et al. Pharmaceuticals (Basel). 2016 Sep 7;9(3):56. doi: 10.3390/ph9030056. Pharmaceuticals (Basel). 2016. PMID: 27618065 Free PMC article. - Comparative Analysis of the Antimicrobial Activities of Plant Defensin-Like and Ultrashort Peptides against Food-Spoiling Bacteria.
Kraszewska J, Beckett MC, James TC, Bond U. Kraszewska J, et al. Appl Environ Microbiol. 2016 Jun 30;82(14):4288-4298. doi: 10.1128/AEM.00558-16. Print 2016 Jul 15. Appl Environ Microbiol. 2016. PMID: 27208129 Free PMC article.
References
- Giuliani A, Pirri G, Nicoletto SF. Antimicrobial peptides: An overview of a promising class of therapeutics. Cent Eur J Biol. 2007;2(1):1–33.
- Mygind PH, et al. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature. 2005;437(7061):975–980. - PubMed
- Schneider T, et al. Plectasin, a fungal defensin, targets the bacterial cell wall precursor Lipid II. Science. 2010;328(5982):1168–1172. - PubMed
- Patra M, Gasser G, Metzler-Nolte N. Small organometallic compounds as antibacterial agents. Dalton Trans. 2012;41(21):6350–6358. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases