PAKs inhibitors ameliorate schizophrenia-associated dendritic spine deterioration in vitro and in vivo during late adolescence - PubMed (original) (raw)
PAKs inhibitors ameliorate schizophrenia-associated dendritic spine deterioration in vitro and in vivo during late adolescence
Akiko Hayashi-Takagi et al. Proc Natl Acad Sci U S A. 2014.
Abstract
Drug discovery in psychiatry has been limited to chemical modifications of compounds originally discovered serendipitously. Therefore, more mechanism-oriented strategies of drug discovery for mental disorders are awaited. Schizophrenia is a devastating mental disorder with synaptic disconnectivity involved in its pathophysiology. Reduction in the dendritic spine density is a major alteration that has been reproducibly reported in the cerebral cortex of patients with schizophrenia. Disrupted-in-Schizophrenia-1 (DISC1), a factor that influences endophenotypes underlying schizophrenia and several other neuropsychiatric disorders, has a regulatory role in the postsynaptic density in association with the NMDA-type glutamate receptor, Kalirin-7, and Rac1. Prolonged knockdown of DISC1 leads to synaptic deterioration, reminiscent of the synaptic pathology of schizophrenia. Thus, we tested the effects of novel inhibitors to p21-activated kinases (PAKs), major targets of Rac1, on synaptic deterioration elicited by knockdown expression of DISC1. These compounds not only significantly ameliorated the synaptic deterioration triggered by DISC1 knockdown but also partially reversed the size of deteriorated synapses in culture. One of these PAK inhibitors prevented progressive synaptic deterioration in adolescence as shown by in vivo two-photon imaging and ameliorated a behavioral deficit in prepulse inhibition in adulthood in a DISC1 knockdown mouse model. The efficacy of PAK inhibitors may have implications in drug discovery for schizophrenia and related neuropsychiatric disorders in general.
Keywords: mechanism-oriented drug discovery; synapse protection.
Conflict of interest statement
Conflict of interest statement: B.V., S.G.D., and D.A.C. are former employees of Afraxis, Inc.
Figures
Fig. 1.
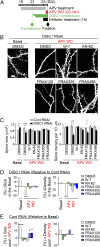
DISC1 knockdown affects NMDA receptor (NMDA-R)-dependent synaptic plasticity. (A) Time lapse imaging showing structural plasticity of spine upon APV withdrawal (APV WD). Whereas spines on neurons with control shRNA (control RNAi, arrowheads) underwent spine enlargement, spines with DISC1 shRNA (DISC1 RNAi, arrows) were reduced in size upon NMDA-R activation. The absolute values of spine size and relative size upon APV WD are shown, which suggests that synaptic response between control and DISC1 RNAi-treated neuron are significantly different. (B) Changes of mEPSC upon APV WD in neurons with or without DISC1 RNAi. **P < 0.01, ***P < 0.001 compared with t = 0 min. (C) Changes of uEPSC showing functional plasticity upon APV WD in neurons with or without DISC1 RNAi. APV withdrawal induced spine enlargement and enhancement in synaptic transmission in control neuron (spines 1 and 2), whereas in contrast, there were spine shrinkage and reduction in synaptic transmission in DISC1 RNAi-treated neuron (spines 3 and 4). Changes in uEPSC amplitude (Δamplitude) in each individual spine are shown (control RNAi, n = 16; DISC1 RNAi, n = 16). ***P < 0.001.
Fig. 2.
Characteristics of PAK inhibitors. Chemical structure of three major compounds (A) and pharmacological properties (B).
Fig. 3.
Chemical inhibitors of PAK block DISC1 knockdown-elicited synaptic changes associated with NMDA-R activation. (A) Experimental design. Inhibitors with concentrations of 10 μM for W-7, 10 μM for KN-62, 250 nM for FRAX355, 500 nM for FRAX120, or 500 nM for FRAX486 were added 1 h before APV WD, followed by incubation of APV WD solution containing the inhibitor at same concentration for 20 min. (B_–_E) Morphometry spine analysis showing the effect of each compound on APV WD-induced spine structural change. The absolute values of spine size and density and the relative size and density after APV WD in the presence of each compound [∆size (%) and ∆density (%)] are shown. Beneficial effects of PAK inhibitors to the shrinkage of spine size were shown. In particular, FRAX355 and FRAX486 displayed beneficial influences on spine density without robustly affecting synaptic plasticity. **P < 0.01. ***P < 0.001. (Scale bar, 5 μm.)
Fig. 4.
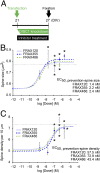
PAK inhibitors prevent DISC1 RNAi-induced spine deterioration (prophylactic effect). (A) Experimental design. PAK inhibitors were added at the time of transfection for DISC1 RNAi and incubated for 6 d before fixation. (B and C) Dose–response curves of FRAX120, FRAX355, and FRAX486 for protective effects on the spine size (B) and spine density (C) in the neurons with DISC1 shRNA (DISC1 RNAi) are shown. Asterisks indicate significant differences between the spines with vehicle control and those with PAK inhibitor, which imply therapeutic effective dose of each compound *P < 0.05. The dotted lines indicate the average of the spine size (B) and the spine density (C) in cells with control shRNA (baselines). “Veh” indicates “Vehicle only” in dose–response curve. _EC_50, half-maximum effective concentration in this prophylactic paradigm, is calculated.
Fig. 5.
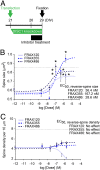
Chemical inhibitors of PAK reverse already existing spine deterioration triggered by DISC1 RNAi (treatment effect). (A) Experimental design. PAK inhibitors were added 5 d after the transfection of either control or DISC1 RNAi, followed by 3 d incubation before fixation. (B and C) Dose–response curves of FRAX120, FRAX355, and FRAX486 for reversal effect on spine size (B) and spine density (C) in the neuron with DISC1 shRNA are shown. Asterisks indicate the significant differences between the spines with vehicle control and those with PAK inhibitor *P < 0.05. The dotted lines indicate the average of the spine size (B) and the spine density (C) in cells with control shRNA (baselines). “Veh” indicates “Vehicle only” in dose–response curve. _EC_50, half-maximum effective concentration in this treatment paradigm, is calculated.
Fig. 6.
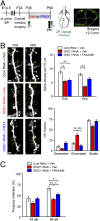
The PAK inhibitor (FRAX486) ameliorates adolescent synapse loss in the prefrontal cortex and adult behavior change in a DISC1 knockdown mouse model. (A) Experimental design. DISC1 or control shRNA was transferred at embryonic day 14.5 (E14.5). Mice with shRNA were subjected to cranial window surgery at postnatal day 34 (P34). Two-photon imaging (2P) was performed both at P35 and P60. Location of cranial window and shRNA distribution (in green) at P60 were shown. Prepulse inhibition (PPI) was tested just before the two-photon imaging at P60. (B) Spine analyses. DISC1 shRNA led to a marked reduction in the spine density at both postnatal days 35 and 60 (P35 and P60), which was further augmented at P60 (**P < 0.01; ***P < 0.001). Daily administration of FRAX486, but not that of vehicle, between P35 and P60 blocked the exacerbated spine loss during adolescence. The effects of FRAX486 were significant in blocking spine elimination (***P < 0.001). In addition, a trend of enhanced spine generation (P = 0.07) was observed by treatment with FRAX486. Spines surrounded by yellow and green dots indicate eliminated (indicated as E) and generated (indicated as G) spines between P35 and P60, respectively. (Scale bar, 2 μm.) (C) Performance in PPI at P60. Deficit in PPI in DISC1-knockdown mice was significantly, although partially, ameliorated by FRAX486 (n = 12 per each group). *P < 0.05, **P < 0.01.
Similar articles
- Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1.
Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, Makino Y, Seshadri AJ, Ishizuka K, Srivastava DP, Xie Z, Baraban JM, Houslay MD, Tomoda T, Brandon NJ, Kamiya A, Yan Z, Penzes P, Sawa A. Hayashi-Takagi A, et al. Nat Neurosci. 2010 Mar;13(3):327-32. doi: 10.1038/nn.2487. Epub 2010 Feb 7. Nat Neurosci. 2010. PMID: 20139976 Free PMC article. - FRAX597, a small molecule inhibitor of the p21-activated kinases, inhibits tumorigenesis of neurofibromatosis type 2 (NF2)-associated Schwannomas.
Licciulli S, Maksimoska J, Zhou C, Troutman S, Kota S, Liu Q, Duron S, Campbell D, Chernoff J, Field J, Marmorstein R, Kissil JL. Licciulli S, et al. J Biol Chem. 2013 Oct 4;288(40):29105-14. doi: 10.1074/jbc.M113.510933. Epub 2013 Aug 19. J Biol Chem. 2013. PMID: 23960073 Free PMC article. - Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by the small-molecule PAK inhibitor FRAX486.
Dolan BM, Duron SG, Campbell DA, Vollrath B, Shankaranarayana Rao BS, Ko HY, Lin GG, Govindarajan A, Choi SY, Tonegawa S. Dolan BM, et al. Proc Natl Acad Sci U S A. 2013 Apr 2;110(14):5671-6. doi: 10.1073/pnas.1219383110. Epub 2013 Mar 18. Proc Natl Acad Sci U S A. 2013. PMID: 23509247 Free PMC article. - Mechanisms underlying the role of DISC1 in synaptic plasticity.
Tropea D, Hardingham N, Millar K, Fox K. Tropea D, et al. J Physiol. 2018 Jul;596(14):2747-2771. doi: 10.1113/JP274330. J Physiol. 2018. PMID: 30008190 Free PMC article. Review.
Cited by
- Neurexin-Neuroligin Synaptic Complex Regulates Schizophrenia-Related DISC1/Kal-7/Rac1 "Signalosome".
Owczarek S, Bang ML, Berezin V. Owczarek S, et al. Neural Plast. 2015;2015:167308. doi: 10.1155/2015/167308. Epub 2015 May 20. Neural Plast. 2015. PMID: 26078884 Free PMC article. - PAK inhibitor FRAX486 decreases the metastatic potential of triple-negative breast cancer cells by blocking autophagy.
Lyu L, Li H, Lu K, Jiang S, Li H. Lyu L, et al. Br J Cancer. 2024 Feb;130(3):394-405. doi: 10.1038/s41416-023-02523-4. Epub 2023 Dec 18. Br J Cancer. 2024. PMID: 38110664 Free PMC article. - Myosin IIA-related Actomyosin Contractility Mediates Oxidative Stress-induced Neuronal Apoptosis.
Wang Y, Xu Y, Liu Q, Zhang Y, Gao Z, Yin M, Jiang N, Cao G, Yu B, Cao Z, Kou J. Wang Y, et al. Front Mol Neurosci. 2017 Mar 14;10:75. doi: 10.3389/fnmol.2017.00075. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28352215 Free PMC article. - Engineering Pak1 Allosteric Switches.
Dagliyan O, Karginov AV, Yagishita S, Gale ME, Wang H, DerMardirossian C, Wells CM, Dokholyan NV, Kasai H, Hahn KM. Dagliyan O, et al. ACS Synth Biol. 2017 Jul 21;6(7):1257-1262. doi: 10.1021/acssynbio.6b00359. Epub 2017 Apr 6. ACS Synth Biol. 2017. PMID: 28365983 Free PMC article. - Morphological and transcriptomic analyses of stem cell-derived cortical neurons reveal mechanisms underlying synaptic dysfunction in schizophrenia.
Kathuria A, Lopez-Lengowski K, Watmuff B, Karmacharya R. Kathuria A, et al. Genome Med. 2023 Jul 28;15(1):58. doi: 10.1186/s13073-023-01203-5. Genome Med. 2023. PMID: 37507766 Free PMC article.
References
- Ibrahim HM, Tamminga CA. Schizophrenia: Treatment targets beyond monoamine systems. Annu Rev Pharmacol Toxicol. 2011;51:189–209. - PubMed
- Bray NJ, Leweke FM, Kapur S, Meyer-Lindenberg A. The neurobiology of schizophrenia: New leads and avenues for treatment. Curr Opin Neurobiol. 2010;20(6):810–815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 MH094268/MH/NIMH NIH HHS/United States
- MH-069853/MH/NIMH NIH HHS/United States
- MH-092443/MH/NIMH NIH HHS/United States
- R01 MH069853/MH/NIMH NIH HHS/United States
- MH-084018/MH/NIMH NIH HHS/United States
- MH-085226/MH/NIMH NIH HHS/United States
- MH-094268/MH/NIMH NIH HHS/United States
- RC1 MH088753/MH/NIMH NIH HHS/United States
- R01 MH092443/MH/NIMH NIH HHS/United States
- P20 MH084018/MH/NIMH NIH HHS/United States
- R01 DA037618/DA/NIDA NIH HHS/United States
- MH-088753/MH/NIMH NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R21 MH085226/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials