Specificity of the transport of lipid II by FtsW in Escherichia coli - PubMed (original) (raw)
Specificity of the transport of lipid II by FtsW in Escherichia coli
Tamimount Mohammadi et al. J Biol Chem. 2014.
Abstract
Synthesis of biogenic membranes requires transbilayer movement of lipid-linked sugar molecules. This biological process, which is fundamental in prokaryotic cells, remains as yet not clearly understood. In order to obtain insights into the molecular basis of its mode of action, we analyzed the structure-function relationship between Lipid II, the important building block of the bacterial cell wall, and its inner membrane-localized transporter FtsW. Here, we show that the predicted transmembrane helix 4 of Escherichia coli FtsW (this protein consists of 10 predicted transmembrane segments) is required for the transport activity of the protein. We have identified two charged residues (Arg(145) and Lys(153)) within this segment that are specifically involved in the flipping of Lipid II. Mutating these two amino acids to uncharged ones affected the transport activity of FtsW. This was consistent with loss of in vivo activity of the mutants, as manifested by their inability to complement a temperature-sensitive strain of FtsW. The transport activity of FtsW could be inhibited with a Lipid II variant having an additional size of 420 Da. Reducing the size of this analog by about 274 Da resulted in the resumption of the transport activity of FtsW. This suggests that the integral membrane protein FtsW forms a size-restricted porelike structure, which accommodates Lipid II during transport across the bacterial cytoplasmic membrane.
Keywords: Antibiotics; Cell Wall; Lipid Transport; Membrane Biogenesis; Phospholipid.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
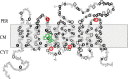
FIGURE 1.
Putative membrane topology model of E. coli FtsW (reprinted with permission from Ref. 3). The generation of FtsW mutants (used in this study) lacking various TM segments was based on this predicted membrane topology model. Red circles, sites where the stop codons were introduced to create mutants lacking TM10, TM7–TM10, TM5–TM10, or TM4–TM10. Green circles, triple alanine substitution mutants in TM4.
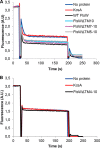
FIGURE 2.
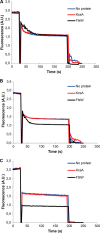
The transport activity of FtsW requires TM4. A, TM5–TM10 are not essential for the transport of NBD-Lipid II. Mutants lacking various segments of FtsW were assessed for transport activity using the dithionite reduction assay. As described under “Materials and Methods,” this was achieved by reconstitution of the purified proteins into LUVs containing NBD-Lipid II symmetrically distributed between inner and outer leaflets of the bilayer. After solubilization with Triton X-100, LUVs were reconstituted with no protein, the control protein KcsA, or FtsW. After the addition of dithionite (1), a reduction of almost 50% of the fluorescence signal is observed in protein-free vesicles and proteoliposomes containing KcsA. On the contrary, about 70% quenching is detectable when vesicles containing wild type FtsW or FtsW lacking TM10 or TM7–TM10 or TM5–TM10 were assayed. When 0.1% Triton X-100 was added (2) to permeabilize the model membranes, a complete quenching of all of the fluorescence is attained. B, TM4 is involved in the transport of NBD-Lipid II. FtsW lacking TM4–TM10 displayed the same effect as the controls (LUVs with no protein or with KcsA) with regard to the extent of quenching by dithionite. All measurements were carried out at 20 °C and are representative of at least three independent experiments. A.U., arbitrary units.
FIGURE 3.
Sequence alignment of the residues Arg145 and Lys153 in the predicted TM4 of FtsW in E. coli and other bacteria using the Clustal version 2.1 multiple sequence alignment program. The residues Arg145 and Lys153 and their homologues are highlighted in green and red, respectively.
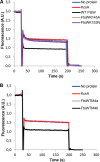
FIGURE 4.
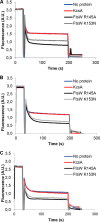
The residues Arg145 and Lys153 within TM4 are essential for the transport activity of FtsW. A, substitution of arginine 145 by alanine abolished the transport activity of FtsW protein. Mutating lysine 153 to asparagine resulted in loss of transport activity of FtsW as well. B, triple alanine substitutions of the amino acids glutamic acid 150, leucine 151, and threonine 152 (TM4a) and the residues leucine 154, serine 155, and leucine 156 (TM4b) (see also Fig. 1 for the putative topology model) did not alter the transport activity of FtsW. The assay was carried out as detailed in the legend to Fig. 2. All measurements were performed at 20 °C and are representative of at least three independent experiments. A.U., arbitrary units.
FIGURE 5.
A, localization of GFP-FtsW fusions in wild-type E. coli LMC500. The constructs containing wild type ftsW and the substitution mutants fused to the C terminus of mut2gfp were transformed to LMC500 and grown at 28 °C in TY medium as described under “Materials and Methods.” The expression of the proteins was induced with 10 μ
m
IPTG for 90 min. Each panel consists of (from left to right) a phase-contrast image, a GFP fluorescence image, and a map of fluorescence profiles of 500- 1000 cells. Scale bar, 5 μm. B, complementation assay of wild-type FtsW and substitution mutants in strain JLB17. The plasmids containing wild type ftsW and the substitution mutants were transformed to JLB17FtsW(Ts) and grown at 28 or 42 °C in TY medium without salt supplemented with thymine as described under “Materials and Methods.” The cells were imaged by phase-contrast microscopy. The image of the FtsW(Ts) cells expressing wild-type FtsW at 42 °C is a composite image to show that in this culture also some filaments were present. Note that the JLB17FtsW(Ts) cells expressing FtsWK153N are thinner than the cells expressing WT FtsW or FtsWR145A. Scale bar, 10 μm.
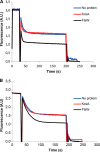
FIGURE 6.
FtsW is able to transport NBD-PE and NBD-PG. LUVs containing NBD-PE (A) or NBD-PG (B) were reconstituted with no protein, KcsA, or FtsW as described before (see details in the legend to Fig. 2).
FIGURE 7.
The residues Arg145 and Lys153 of FtsW are not important for the transport of NBD-PL. Substitution mutants R145A and K153N did not affect the ability of FtsW to transport NBD-PE (A) or NBD-PG (B) in model membranes. The flippase activity of FtsW R145A and FtsW K153N was shown to be independent of the headgroup of the phospholipid because these mutants were able to facilitate the transbilayer movement of NBD-PC (C). The assay was carried out as detailed in the legend to Fig. 2.
FIGURE 8.
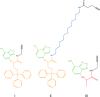
Structure of compounds I, II, and III used to synthesize Lipid II analogues 1, 2, and 3, respectively. These NBD-amino acid-alkyne compounds were designed and labeled as described under “Materials and Methods.” The NBD group is drawn in green. The cysteine (trityl) backbone is highlighted in orange. The PEG linker in compound II is highlighted in blue, and the Ala backbone in compound III is marked in red.
FIGURE 9.
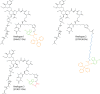
Structures of Lipid II analogues used in this study. The analogues were synthesized as described under “Materials and Methods.” In these analogues, the NBD fluorophore is highlighted in green. The cysteine (trityl) backbone in analog 1 and 2 is marked in orange. The PEG linker in analog 2 is highlighted in blue, and the Ala backbone in analog 3 is marked in red.
FIGURE 10.
Inhibition of Lipid II analog 1 transport activity of FtsW. In LUVs containing analog 1, the transport was blocked (A), whereas FtsW retained its transport activity in the presence of analog 2 (B). When the size of analog 1 was reduced by about 274 Da (resulting in analog 3), FtsW allowed the passage of this latter (C). The assay was performed as detailed in the legend to Fig. 2.
FIGURE 11.
Schematic illustrating that Lipid II transport by FtsW requires restricted size and flexibility of the substrate. FtsW allowed the transport of analog 3, whereas analog 1 that is 247 Da (in molecular mass) bigger than analog 3 could not be transported. This suggests that transport by FtsW possibly occurs through a porelike structure with a limited size. The ability of FtsW to transport analog 2, which is 245 Da larger than analog 1, demonstrates that flexibility of the substrate is important; the presence of the PEG linker in analog 2 (see also Fig. 9) makes it more flexible to move between the leaflets of the membrane, whereas analog 1 (deprived of the linker) is more compact. The additional groups in Lipid II analogues are illustrated by pink spheres.
Similar articles
- Interplay between Penicillin-binding proteins and SEDS proteins promotes bacterial cell wall synthesis.
Leclercq S, Derouaux A, Olatunji S, Fraipont C, Egan AJ, Vollmer W, Breukink E, Terrak M. Leclercq S, et al. Sci Rep. 2017 Feb 24;7:43306. doi: 10.1038/srep43306. Sci Rep. 2017. PMID: 28233869 Free PMC article. - Identification of FtsW as a transporter of lipid-linked cell wall precursors across the membrane.
Mohammadi T, van Dam V, Sijbrandi R, Vernet T, Zapun A, Bouhss A, Diepeveen-de Bruin M, Nguyen-Distèche M, de Kruijff B, Breukink E. Mohammadi T, et al. EMBO J. 2011 Apr 20;30(8):1425-32. doi: 10.1038/emboj.2011.61. Epub 2011 Mar 8. EMBO J. 2011. PMID: 21386816 Free PMC article. - FtsW activity and lipid II synthesis are required for recruitment of MurJ to midcell during cell division in Escherichia coli.
Liu X, Meiresonne NY, Bouhss A, den Blaauwen T. Liu X, et al. Mol Microbiol. 2018 Sep;109(6):855-884. doi: 10.1111/mmi.14104. Epub 2018 Sep 26. Mol Microbiol. 2018. PMID: 30112777 - Class A PBPs: It is time to rethink traditional paradigms.
Straume D, Piechowiak KW, Kjos M, Håvarstein LS. Straume D, et al. Mol Microbiol. 2021 Jul;116(1):41-52. doi: 10.1111/mmi.14714. Epub 2021 Mar 23. Mol Microbiol. 2021. PMID: 33709487 Review. - The Bacterial Cell Wall: From Lipid II Flipping to Polymerization.
Kumar S, Mollo A, Kahne D, Ruiz N. Kumar S, et al. Chem Rev. 2022 May 11;122(9):8884-8910. doi: 10.1021/acs.chemrev.1c00773. Epub 2022 Mar 11. Chem Rev. 2022. PMID: 35274942 Free PMC article. Review.
Cited by
- Cell wall peptidoglycan in Mycobacterium tuberculosis: An Achilles' heel for the TB-causing pathogen.
Maitra A, Munshi T, Healy J, Martin LT, Vollmer W, Keep NH, Bhakta S. Maitra A, et al. FEMS Microbiol Rev. 2019 Sep 1;43(5):548-575. doi: 10.1093/femsre/fuz016. FEMS Microbiol Rev. 2019. PMID: 31183501 Free PMC article. Review. - The transpeptidase PbpA and noncanonical transglycosylase RodA of Mycobacterium tuberculosis play important roles in regulating bacterial cell lengths.
Arora D, Chawla Y, Malakar B, Singh A, Nandicoori VK. Arora D, et al. J Biol Chem. 2018 Apr 27;293(17):6497-6516. doi: 10.1074/jbc.M117.811190. Epub 2018 Mar 12. J Biol Chem. 2018. PMID: 29530985 Free PMC article. - Structural basis for the coordination of cell division with the synthesis of the bacterial cell envelope.
Booth S, Lewis RJ. Booth S, et al. Protein Sci. 2019 Dec;28(12):2042-2054. doi: 10.1002/pro.3722. Epub 2019 Sep 30. Protein Sci. 2019. PMID: 31495975 Free PMC article. Review. - The coiled-coil domain of Escherichia coli FtsLB is a structurally detuned element critical for modulating its activation in bacterial cell division.
Craven SJ, Condon SGF, Díaz Vázquez G, Cui Q, Senes A. Craven SJ, et al. J Biol Chem. 2022 Jan;298(1):101460. doi: 10.1016/j.jbc.2021.101460. Epub 2021 Dec 4. J Biol Chem. 2022. PMID: 34871549 Free PMC article. - Bacteria can anticipate the seasons: photoperiodism in cyanobacteria.
Jabbur ML, Johnson CH. Jabbur ML, et al. bioRxiv [Preprint]. 2024 Aug 8:2024.05.13.593996. doi: 10.1101/2024.05.13.593996. bioRxiv. 2024. PMID: 38798677 Free PMC article. Updated. Preprint.
References
- Egan A. J., Vollmer W. (2013) The physiology of bacterial cell division. Ann. N.Y. Acad. Sci. 1277, 8–28 - PubMed
- Lara B., Ayala J. A. (2002) Topological characterization of the essential Escherichia coli cell division protein FtsW. FEMS Microbiol. Lett. 216, 23–32 - PubMed
- Fraipont C., Alexeeva S., Wolf B., van der Ploeg R., Schloesser M., den Blaauwen T., Nguyen-Distèche M. (2011) The integral membrane FtsW protein and peptidoglycan synthase PBP3 form a sbucomplex in Escherichia coli. Microbiology 157, 251–259 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases