Investigating G protein signalling bias at the glucagon-like peptide-1 receptor in yeast - PubMed (original) (raw)
Investigating G protein signalling bias at the glucagon-like peptide-1 receptor in yeast
C Weston et al. Br J Pharmacol. 2014 Aug.
Abstract
Background and purpose: The glucagon-like peptide 1 (GLP-1) receptor performs an important role in glycaemic control, stimulating the release of insulin. It is an attractive target for treating type 2 diabetes. Recently, several reports of adverse side effects following prolonged use of GLP-1 receptor therapies have emerged: most likely due to an incomplete understanding of signalling complexities.
Experimental approach: We describe the expression of the GLP-1 receptor in a panel of modified yeast strains that couple receptor activation to cell growth via single Gα/yeast chimeras. This assay enables the study of individual ligand-receptor G protein coupling preferences and the quantification of the effect of GLP-1 receptor ligands on G protein selectivity.
Key results: The GLP-1 receptor functionally coupled to the chimeras representing the human Gαs, Gαi and Gαq subunits. Calculation of the dissociation constant for a receptor antagonist, exendin-3 revealed no significant difference between the two systems. We obtained previously unobserved differences in G protein signalling bias for clinically relevant therapeutic agents, liraglutide and exenatide; the latter displaying significant bias for the Gαi pathway. We extended the use of the system to investigate small-molecule allosteric compounds and the closely related glucagon receptor.
Conclusions and implications: These results provide a better understanding of the molecular events involved in GLP-1 receptor pleiotropic signalling and establish the yeast platform as a robust tool to screen for more selective, efficacious compounds acting at this important class of receptors in the future.
Keywords: G proteins; GPCR; diabetes; exenatide; glucagon; glucagon-like peptide-1 (GLP-1); liraglutide; signal bias.
© 2014 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of The British Pharmacological Society.
Figures
Figure 1
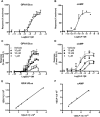
Activation of the yeast-mating pathway by the GLP-1 receptor. (A) Strains containing the GLP-1 receptor were stimulated with 0 or 10 μM GLP-1 for 20 h and assayed for activation of the FUS1 > lacZ reporter gene. Data are mean of five independent experiments ± SEM. Data were determined as significantly different from the non-peptide response using Student's _t_-test where **P < 0.01, ***P < 0.001. (B) Expression of the GLP-1 receptor in yeast strains containing various GPA1/Gα chimeras was confirmed using immunoblotting. (C) A C-terminal 3xmCherry tag was engineered onto the GLP-1 receptor and expression at the plasma membrane was confirmed using fluorescence microscopy. Scale bar = 10 μm.
Figure 2
The GPA1/Gαs responses reproduce cAMP data for GLP-1 receptor agonism. (A) Dose-response curves to the natural GLP-1 receptor agonist, GLP-1, were constructed in the yeast strain containing the GPA1/Gαs chimera. Activation of the reporter gene was calculated as a percentage of the maximum response observed. (B) cAMP accumulation was determined following 30 min stimulation of transiently transfected HEK293T cells and expressed as a percentage of the maximum observed. (C) S . cerevisiae containing the GPA1/Gαs chimera and (D) transiently transfected HEK293T cells were stimulated with GLP-1 in the presence of the indicated concentrations of exendin-3. All data are expressed as a percentage of the maximal response in the absence of inhibitor and are mean of 5–8 independent experiments ± SEM. (E) and (F) Double reciprocal plots for GLP-1 in the presence (Y axis) and absence (X axis) of 10 nM exendin-3.
Figure 3
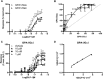
The G protein chimera influences the activity profile of GLP-1 receptor agonist. (A) Dose-response curves to the natural GLP-1 receptor agonist, GLP-1 were constructed in strains containing the GPA1/Gαs or GPA1/Gαi chimera. Activation of the reporter gene was calculated as a percentage of the maximum response observed in the GPA1/Gαs strain. (B) Equimolar comparison of bias, the normalized response at equivalent concentrations of agonist for each G protein chimera shown in (A) was plotted to allow visualization of any pathway preference. Dashed line represents no bias. (C) GPA1/Gαi chimera strain was stimulated with GLP-1 in the presence of the indicated concentrations of exendin-3. Reporter gene activity was determined following 20 h stimulation. All data are expressed as a percentage of the maximal response in the absence of inhibitor and are mean of 5–8 independent experiments ± SEM. (D) Double reciprocal plot for GLP-1 in the presence (Y axis) and absence (X axis) of 10 nM exendin-3.
Figure 4
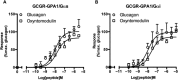
Functional coupling of the glucagon receptor to GPA1/Gαs and GPA1/Gαi. Dose-response curves to glucagon and oxyntomodulin at the glucagon receptor were constructed in yeast strains containing (A), the GPA1/Gαs and (B), the GPA1/Gαi chimera. Activation of the reporter gene was calculated as a percentage of the maximum response observed.
Figure 5
Activation of the GLP-1 receptor by glucagon and oxyntomodulin. (A) Yeast strains expressing the GLP-1 receptor were stimulated with GLP-1, glucagon or oxyntomodulin for 20 h and reporter gene activity determined following coupling of the receptor to (A) the GPA1/Gαs or (B) the GPA1/Gαi chimera. Data are expressed as a % of the maximum response achieved by GLP-1 in the GPA1/Gαs strain. (C) The normalized responses for equivalent concentrations of each ligand were plotted to observe the relative G protein bias where the dashed line represents no bias. (D) Signalling bias was calculated relative to GLP-1 as the change in log(τ/_K_A) ratio for the data in (A) and (B). Data were determined as statistically different (**P < 0.01, ***P < 0.001) from GLP-1, using a one-way
anova
with Bonferroni's post-test. All data are mean of 5–8 independent experiments ± SEM.
Figure 6
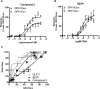
Activation of the GLP-1 receptor by non-peptide ligands. Yeast strains expressing the GLP-1 receptor containing either the GPA1/Gαs or GPA1/Gαi chimera stimulated with a range of (A), compound 2 or (B), BETP concentrations for 20 h and reporter gene activity determined. Data expressed as a percentage of the maximum response achieved through activation of GPA1/Gαs. (C) To observe pathway preferences for each ligand, the normalized responses for equivalent concentrations of ligand were used to generate an equimolar bias plot; the dashed line represents no bias. All data are mean of five independent experiments ± SEM.
Figure 7
The influence of G protein subtype on GLP-1 receptor activation by peptide drugs used in the treatment of type 2 diabetes. (A) Sequence homology of GLP-1 mimetics. Blue circles are conserved throughout all three peptides, orange circles are unique to liraglutide and red denotes exenatide residues. The DPP-IV cleavage site is indicated by the dashed line. (B, C) Concentration-response curves were constructed to each of the GLP-1 receptor agonists in the indicated yeast strains. Each strain was assayed for reporter gene activity following incubation with a range of ligand concentrations for 20 h. Data are presented as the % maximal response achieved by GLP-1 at the GPA1/Gαs chimera strain. (D) Equimolar bias comparison generated by plotting the normalized responses at each G protein chimera for equivalent concentrations of ligand. Dashed line represents no bias. (E) The signalling bias was determined for each drug relative to the natural agonist using the change in log(τ/_K_A) ratio for the data in (B) and (C). Statistical significance was determined using one-way
anova
with Bonferroni's post-test with each data set compared with GLP-1 (***P < 0.001). All data are mean of 5–8 independent experiments ± SEM.
Figure 8
Comparison of GLP-1 receptor peptide ligands and relative bias factors. Sequences of the various peptide ligands for the GLP-1 receptor aligned to the potent, natural agonist. Amino acids differing from those in GLP-1 are highlighted in grey. The relative (to GLP-1) bias factor was quantified for each ligand as the change in log(τ/_K_A) ratio where a negative value indicates preference for the inhibitory, Gαi chimera. Statistical significance was determined using one-way
anova
with Bonferroni's post-test with each data set compared with GLP-1 (**P < 0.01, ***P < 0.001). Data are mean of 5–8 independent experiments ± SEM.
Similar articles
- Proinflammatory switch from Gαs to Gαi signaling by Glucagon-like peptide-1 receptor in murine splenic monocyte following burn injury.
Zhang QH, Hao JW, Li GL, Ji XJ, Yao XD, Dong N, Yao YM. Zhang QH, et al. Inflamm Res. 2018 Feb;67(2):157-168. doi: 10.1007/s00011-017-1104-9. Epub 2017 Oct 11. Inflamm Res. 2018. PMID: 29022064 - Positive Allosteric Modulation of the Glucagon-like Peptide-1 Receptor by Diverse Electrophiles.
Bueno AB, Showalter AD, Wainscott DB, Stutsman C, Marín A, Ficorilli J, Cabrera O, Willard FS, Sloop KW. Bueno AB, et al. J Biol Chem. 2016 May 13;291(20):10700-15. doi: 10.1074/jbc.M115.696039. Epub 2016 Mar 14. J Biol Chem. 2016. PMID: 26975372 Free PMC article. - Modulation of Glucagon Receptor Pharmacology by Receptor Activity-modifying Protein-2 (RAMP2).
Weston C, Lu J, Li N, Barkan K, Richards GO, Roberts DJ, Skerry TM, Poyner D, Pardamwar M, Reynolds CA, Dowell SJ, Willars GB, Ladds G. Weston C, et al. J Biol Chem. 2015 Sep 18;290(38):23009-22. doi: 10.1074/jbc.M114.624601. Epub 2015 Jul 21. J Biol Chem. 2015. PMID: 26198634 Free PMC article. - GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art.
Nauck MA, Quast DR, Wefers J, Meier JJ. Nauck MA, et al. Mol Metab. 2021 Apr;46:101102. doi: 10.1016/j.molmet.2020.101102. Epub 2020 Oct 14. Mol Metab. 2021. PMID: 33068776 Free PMC article. Review. - Molecular mechanisms underlying physiological and receptor pleiotropic effects mediated by GLP-1R activation.
Pabreja K, Mohd MA, Koole C, Wootten D, Furness SG. Pabreja K, et al. Br J Pharmacol. 2014 Mar;171(5):1114-28. doi: 10.1111/bph.12313. Br J Pharmacol. 2014. PMID: 23889512 Free PMC article. Review.
Cited by
- Expression and purification of recombinant G protein-coupled receptors: A review.
Wiseman DN, Otchere A, Patel JH, Uddin R, Pollock NL, Routledge SJ, Rothnie AJ, Slack C, Poyner DR, Bill RM, Goddard AD. Wiseman DN, et al. Protein Expr Purif. 2020 Mar;167:105524. doi: 10.1016/j.pep.2019.105524. Epub 2019 Oct 31. Protein Expr Purif. 2020. PMID: 31678667 Free PMC article. Review. - Functional expression of opioid receptors and other human GPCRs in yeast engineered to produce human sterols.
Bean BDM, Mulvihill CJ, Garge RK, Boutz DR, Rousseau O, Floyd BM, Cheney W, Gardner EC, Ellington AD, Marcotte EM, Gollihar JD, Whiteway M, Martin VJJ. Bean BDM, et al. Nat Commun. 2022 May 24;13(1):2882. doi: 10.1038/s41467-022-30570-7. Nat Commun. 2022. PMID: 35610225 Free PMC article. - Loss of dorsomedial hypothalamic GLP-1 signaling reduces BAT thermogenesis and increases adiposity.
Lee SJ, Sanchez-Watts G, Krieger JP, Pignalosa A, Norell PN, Cortella A, Pettersen KG, Vrdoljak D, Hayes MR, Kanoski SE, Langhans W, Watts AG. Lee SJ, et al. Mol Metab. 2018 May;11:33-46. doi: 10.1016/j.molmet.2018.03.008. Epub 2018 Mar 21. Mol Metab. 2018. PMID: 29650350 Free PMC article. - A Hydrogen-Bonded Polar Network in the Core of the Glucagon-Like Peptide-1 Receptor Is a Fulcrum for Biased Agonism: Lessons from Class B Crystal Structures.
Wootten D, Reynolds CA, Koole C, Smith KJ, Mobarec JC, Simms J, Quon T, Coudrat T, Furness SG, Miller LJ, Christopoulos A, Sexton PM. Wootten D, et al. Mol Pharmacol. 2016 Mar;89(3):335-47. doi: 10.1124/mol.115.101246. Epub 2015 Dec 23. Mol Pharmacol. 2016. PMID: 26700562 Free PMC article. - Receptor Activity-modifying Protein-directed G Protein Signaling Specificity for the Calcitonin Gene-related Peptide Family of Receptors.
Weston C, Winfield I, Harris M, Hodgson R, Shah A, Dowell SJ, Mobarec JC, Woodlock DA, Reynolds CA, Poyner DR, Watkins HA, Ladds G. Weston C, et al. J Biol Chem. 2016 Oct 14;291(42):21925-21944. doi: 10.1074/jbc.M116.751362. Epub 2016 Aug 26. J Biol Chem. 2016. PMID: 27566546 Free PMC article.
References
- Anderson SL, Trujillo JM. Association of pancreatitis with glucagon-like peptide-1 agonist use. Ann Pharmacother. 2010;44:904–909. - PubMed
- Baggio LL, Drucker DJ. Biology of Incretins: GLP-1 and GIP. Gastroenterology. 2007;32:2131–2157. - PubMed
- Black JW, Leff P. Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci. 1983;220:141–162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases