Inhibitor of apoptosis-stimulating protein of p53 (iASPP) is required for neuronal survival after axonal injury - PubMed (original) (raw)
Inhibitor of apoptosis-stimulating protein of p53 (iASPP) is required for neuronal survival after axonal injury
Ariel M Wilson et al. PLoS One. 2014.
Abstract
The transcription factor p53 mediates the apoptosis of post-mitotic neurons exposed to a wide range of stress stimuli. The apoptotic activity of p53 is tightly regulated by the apoptosis-stimulating proteins of p53 (ASPP) family members: ASPP1, ASPP2 and iASPP. We previously showed that the pro-apoptotic members ASPP1 and ASPP2 contribute to p53-dependent death of retinal ganglion cells (RGCs). However, the role of the p53 inhibitor iASPP in the central nervous system (CNS) remains to be elucidated. To address this, we asked whether iASPP contributes to the survival of RGCs in an in vivo model of acute optic nerve damage. We demonstrate that iASPP is expressed by injured RGCs and that iASPP phosphorylation at serine residues, which increase iASPP affinity towards p53, is significantly reduced following axotomy. We show that short interference RNA (siRNA)-induced iASPP knockdown exacerbates RGC death, whereas adeno-associated virus (AAV)-mediated iASPP expression promotes RGC survival. Importantly, our data also demonstrate that increasing iASPP expression in RGCs downregulates p53 activity and blocks the expression of pro-apoptotic targets PUMA and Fas/CD95. This study demonstrates a novel role for iASPP in the survival of RGCs, and provides further evidence of the importance of the ASPP family in the regulation of neuronal loss after axonal injury.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
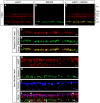
Figure 1. Adult RGCs express iASPP.
Endogenous retinal iASPP was detected by immunofluorescence in the ganglion cell layer (GCL) and inner nuclear layer (INL) (A, C, D, and G). iASPP staining in RGCs was confirmed using the RGC-specific marker RBPMS (D–F). iASPP was also detected in amacrine and horizontal cells, visualized with calretinin (H,J, arrows) and calbindin (I,J, arrows), respectively. Scale bars: (A–C) = 50 μm; (D–J) = 20 μm. PS: Photoreceptor Segments; ONL: Outer Nuclear Layer; OPL: Outer Plexiform Layer; INL: Inner Nuclear Layer; IPL: Inner Plexiform Layer; GCL: Ganglion Cell Layer.
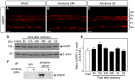
Figure 2. iASPP protein and phosphoserine levels after axotomy.
Retinal iASPP expression and localization did not change at 24 hrs or 3 days after optic nerve injury compared to intact eyes (A–C). Scale bar: 10 μm. Analysis of protein homogenates from axotomized retinas collected at 6, 12, 24, 48 hrs, 3 and 5 days confirmed that iASPP levels were similar to those in intact, non-injured retinas. The lower panel represents the same blot as in the upper panel but probed with an antibody that recognizes β-actin used to confirm equal protein loading (D). Densitometric analysis of western blots, showing the ratio of iASPP protein relative to β-actin, confirmed that there is no significant change in protein levels after injury (E) (ANOVA, p>0.05). Phosphoserine immunoprecipitation (IP) of intact and axotomized retinas probed with iASPP antibody revealed a decrease in phosphoserine iASPP at 24 hrs after axotomy. IP of retinal homogenates with an IgG antibody was included as control for non-specific interactions (F). ONL: Outer Nuclear Layer; OPL: Outer Plexiform Layer; INL: Inner Nuclear Layer; IPL: Inner Plexiform Layer; GCL: Ganglion Cell Layer.
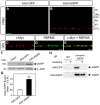
Figure 3. Selective siRNA knockdown of iASPP exacerbates axotomy-induced RGC death.
A significant reduction of iASPP in the GCL was observed by immunohistochemistry of axotomized retinas at 24 hrs after intravitreal delivery of siRNA against iASPP (si-iASPP) compared to intact retinas, while control siRNA against GFP (siGFP) had no effect (A–C). RBPMS labeling confirmed that siRNA-mediated knockdown of iASPP occurred in RGCs (D–F). Scale bars: (A–C) = 50 μm; (D–F) = 15 μm. Western blot analysis confirmed that intravitreal delivery of si-iASPP led to marked reduction of retinal iASPP protein at 24 hrs after delivery, while siGFP had no effect (G, H; Student's T-test, *** = p<0.001). Quantitative analysis of RGC survival at one week after axotomy following intraocular injection of si-iASPP (black), or control siGFP (grey) (n = 4/group, ANOVA, * = p<0.05). The density of RGCs in intact, uninjured Sprague-Dawley rat retinas with si-iASPP intravitreal injection (hatched bar) or without (open bar) are shown as reference. Data are expressed as the mean ± S.E.M. ONL: Outer Nuclear Layer; OPL: Outer Plexiform Layer; INL: Inner Nuclear Layer; IPL: Inner Plexiform Layer; GCL: Ganglion Cell Layer.
Figure 4. Targeted overexpression of iASPP in RGCs increases iASPP phosphoserine levels post-axotomy.
AAV-mediated iASPP expression was distinguished from endogenous iASPP with an antibody against the c-myc tag encoded only in iASPP transgenes. Robust c-myc labeling was observed in the GCL of retinas that received AAV.iASPP, but not in control eyes injected with AAV.GFP (A, B). Selective expression of AAV-mediated iASPP in RGCs was confirmed using the RGC marker RBPMS (C–E). Scale bars: (A–B) = 50 μm; (C–E) = 15 μm. Immunoblotting and densitometric analyses confirmed that intravitreal delivery of AAV.iASPP led to significant overexpression of iASPP protein while AAV.GFP had no effect (F, G; Student's T-test, *** = p<0.001). Phosphoserine immunoprecipitation of retinas probed with an iASPP antibody reveals abundant iASPP phosphoserine levels in axotomized retinas (24 hrs) treated with AAV.iASPP but not with control AAV.GFP (H). PS: Photoreceptor Segments; ONL: Outer Nuclear Layer; OPL: Outer Plexiform Layer; INL: Inner Nuclear Layer; IPL: Inner Plexiform Layer; GCL: Ganglion Cell Layer.
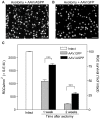
Figure 5. AAV-mediated iASPP overexpression increases RGC survival.
Brn3a-labeled flat-mounted retinas from axotomized eyes demonstrate higher RGC densities following treatment with AAV.iASPP (A) than with AAV.GFP (B) at one week post-injury. Scale bars: 100 μm. Quantitative analysis of RGC survival following axotomy and intraocular injection of AAV.iASPP (black) or control AAV.GFP (grey) (ANOVA, *** = p<0.001) at one and two weeks post-injury (C). The density of RGCs in intact, uninjured Sprague-Dawley rat retinas is shown as reference (open bar). Data are expressed as the mean ± S.E.M.
Figure 6. AAV.iASPP inhibits p53 activation and downregulates retinal PUMA and Fas/CD95 levels.
Western blot analysis of axotomized retinal samples show that p53 phosphoserine15 (pSer15) levels are reduced in AAV.iASPP-treated retinas compared to control AAV.GFP at 24 hrs post-axotomy (A, C; ANOVA, * = p<0.05). Acetyl p53 levels remained unchanged (A, B; ANOVA, p>0.05). The p53 apoptotic targets PUMA and Fas/CD95 protein levels decrease in retinas treated with AAV.iASPP compared to AAV.GFP-treated control retinas (D, E, F; ANOVA, *** = p<0.005, ** = p<0.001), whereas Bax and Noxa remained unchanged (D, G, H; ANOVA, p>0.05).
Similar articles
- ASPP1/2 regulate p53-dependent death of retinal ganglion cells through PUMA and Fas/CD95 activation in vivo.
Wilson AM, Morquette B, Abdouh M, Unsain N, Barker PA, Feinstein E, Bernier G, Di Polo A. Wilson AM, et al. J Neurosci. 2013 Jan 30;33(5):2205-16. doi: 10.1523/JNEUROSCI.2635-12.2013. J Neurosci. 2013. PMID: 23365256 Free PMC article. - Abnormal expression pattern of the ASPP family of proteins in human non-small cell lung cancer and regulatory functions on apoptosis through p53 by iASPP.
Li S, Shi G, Yuan H, Zhou T, Zhang Q, Zhu H, Wang X. Li S, et al. Oncol Rep. 2012 Jul;28(1):133-40. doi: 10.3892/or.2012.1778. Epub 2012 Apr 23. Oncol Rep. 2012. PMID: 22552744 - Extracellular signal-regulated kinase 1/2 mediates survival, but not axon regeneration, of adult injured central nervous system neurons in vivo.
Pernet V, Hauswirth WW, Di Polo A. Pernet V, et al. J Neurochem. 2005 Apr;93(1):72-83. doi: 10.1111/j.1471-4159.2005.03002.x. J Neurochem. 2005. PMID: 15773907 - ASPP and iASPP: Implication in cancer development and progression.
Li Y, Ahmad A, Sarkar FH. Li Y, et al. Cell Mol Biol (Noisy-le-grand). 2015 Oct 30;61(6):2-8. Cell Mol Biol (Noisy-le-grand). 2015. PMID: 26518890 Review. - Reactivating p53 functions by suppressing its novel inhibitor iASPP: a potential therapeutic opportunity in p53 wild-type tumors.
Dong P, Ihira K, Hamada J, Watari H, Yamada T, Hosaka M, Hanley SJ, Kudo M, Sakuragi N. Dong P, et al. Oncotarget. 2015 Aug 21;6(24):19968-75. doi: 10.18632/oncotarget.4847. Oncotarget. 2015. PMID: 26343523 Free PMC article. Review.
Cited by
- Insulin restores retinal ganglion cell functional connectivity and promotes visual recovery in glaucoma.
El Hajji S, Shiga Y, Belforte N, Solorio YC, Tastet O, D'Onofrio P, Dotigny F, Prat A, Arbour N, Fortune B, Di Polo A. El Hajji S, et al. Sci Adv. 2024 Aug 9;10(32):eadl5722. doi: 10.1126/sciadv.adl5722. Epub 2024 Aug 7. Sci Adv. 2024. PMID: 39110798 Free PMC article. - Inhibitor of apoptosis stimulating protein of p53 protects against MPP+-induced neurotoxicity of dopaminergic neurons.
Chen L, Duan F, Ge F, Tian L, Li Y, Li Y, Zhu Q, Zhou Q, Lin H. Chen L, et al. Metab Brain Dis. 2024 Jun;39(5):871-884. doi: 10.1007/s11011-024-01367-y. Epub 2024 Jun 6. Metab Brain Dis. 2024. PMID: 38842662 - Lissencephaly-1 mutations enhance traumatic brain injury outcomes in Drosophila.
Katzenberger RJ, Ganetzky B, Wassarman DA. Katzenberger RJ, et al. Genetics. 2023 Mar 2;223(3):iyad008. doi: 10.1093/genetics/iyad008. Genetics. 2023. PMID: 36683334 Free PMC article. - Pericyte dysfunction and loss of interpericyte tunneling nanotubes promote neurovascular deficits in glaucoma.
Alarcon-Martinez L, Shiga Y, Villafranca-Baughman D, Belforte N, Quintero H, Dotigny F, Cueva Vargas JL, Di Polo A. Alarcon-Martinez L, et al. Proc Natl Acad Sci U S A. 2022 Feb 15;119(7):e2110329119. doi: 10.1073/pnas.2110329119. Proc Natl Acad Sci U S A. 2022. PMID: 35135877 Free PMC article. - Magnesium acetyltaurate prevents retinal damage and visual impairment in rats through suppression of NMDA-induced upregulation of NF-κB, p53 and AP-1 (c-Jun/c-Fos).
Lambuk L, Iezhitsa I, Agarwal R, Agarwal P, Peresypkina A, Pobeda A, Ismail NM. Lambuk L, et al. Neural Regen Res. 2021 Nov;16(11):2330-2344. doi: 10.4103/1673-5374.310691. Neural Regen Res. 2021. PMID: 33818520 Free PMC article.
References
- Bergamaschi D, Samuels Y, O'Neil NJ, Trigiante G, Crook T, et al. (2003) iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. Nat Genet 33: 162–167. - PubMed
- Yang JP, Hori M, Sanda T, Okamoto T (1999) Identification of a novel inhibitor of nuclear factor-kappaB, RelA-associated inhibitor. J Biol Chem 274: 15662–15670. - PubMed
- Slee E, Gillotin Sb, Bergamaschi D, Royer C, Llanos S, et al. (2004) The N-terminus of a novel isoform of human iASPP is required for its cytoplasmic localization. Oncogene 23: 9007–9016. - PubMed
- Ao Y, Rohde LH, Naumovski L (2001) p53-interacting protein 53BP2 inhibits clonogenic survival and sensitizes cells to doxorubicin but not paclitaxel-induced apoptosis. Oncogene 20: 2720–2725. - PubMed
- Bergamaschi D, Samuels Y, Sullivan A, Zvelebil M, Breyssens H, et al. (2006) iASPP preferentially binds p53 proline-rich region and modulates apoptotic function of codon 72-polymorphic p53. Nat Genet 38: 1133–1141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY004067/EY/NEI NIH HHS/United States
- EY021721/EY/NEI NIH HHS/United States
- I01 BX000764/BX/BLRD VA/United States
- VIH-105439/CAPMC/ CIHR/Canada
- P30 EY021721/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous