Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity - PubMed (original) (raw)
. 2014 Apr 10;508(7495):258-62.
doi: 10.1038/nature13198.
Qin Yang 1, Dong Kong 2, Alexander S Banks 3, Lin Zhang 2, Joseph T Rodgers 3, Eija Pirinen 4, Thomas C Pulinilkunnil 5, Fengying Gong 5, Ya-chin Wang 2, Yana Cen 6, Anthony A Sauve 6, John M Asara 7, Odile D Peroni 2, Brett P Monia 8, Sanjay Bhanot 8, Leena Alhonen 4, Pere Puigserver 3, Barbara B Kahn 2
Affiliations
- PMID: 24717514
- PMCID: PMC4107212
- DOI: 10.1038/nature13198
Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity
Daniel Kraus et al. Nature. 2014.
Abstract
In obesity and type 2 diabetes, Glut4 glucose transporter expression is decreased selectively in adipocytes. Adipose-specific knockout or overexpression of Glut4 alters systemic insulin sensitivity. Here we show, using DNA array analyses, that nicotinamide N-methyltransferase (Nnmt) is the most strongly reciprocally regulated gene when comparing gene expression in white adipose tissue (WAT) from adipose-specific Glut4-knockout or adipose-specific Glut4-overexpressing mice with their respective controls. NNMT methylates nicotinamide (vitamin B3) using S-adenosylmethionine (SAM) as a methyl donor. Nicotinamide is a precursor of NAD(+), an important cofactor linking cellular redox states with energy metabolism. SAM provides propylamine for polyamine biosynthesis and donates a methyl group for histone methylation. Polyamine flux including synthesis, catabolism and excretion, is controlled by the rate-limiting enzymes ornithine decarboxylase (ODC) and spermidine-spermine N(1)-acetyltransferase (SSAT; encoded by Sat1) and by polyamine oxidase (PAO), and has a major role in energy metabolism. We report that NNMT expression is increased in WAT and liver of obese and diabetic mice. Nnmt knockdown in WAT and liver protects against diet-induced obesity by augmenting cellular energy expenditure. NNMT inhibition increases adipose SAM and NAD(+) levels and upregulates ODC and SSAT activity as well as expression, owing to the effects of NNMT on histone H3 lysine 4 methylation in adipose tissue. Direct evidence for increased polyamine flux resulting from NNMT inhibition includes elevated urinary excretion and adipocyte secretion of diacetylspermine, a product of polyamine metabolism. NNMT inhibition in adipocytes increases oxygen consumption in an ODC-, SSAT- and PAO-dependent manner. Thus, NNMT is a novel regulator of histone methylation, polyamine flux and NAD(+)-dependent SIRT1 signalling, and is a unique and attractive target for treating obesity and type 2 diabetes.
Figures
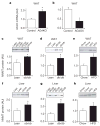
Figure 1. NNMT expression is increased in WAT and liver of obese and insulin-resistant mice
a, b, Nnmt mRNA expression normalized by cyclophilin in WAT of adipose-specific Glut4 knockout (AG4KO) mice and aP2-Cre controls (n =4 per group) (a) and adipose-specific Glut4 overexpressing (AG4OX) and wild-type littermate controls (n =6 per group) (b). c–e, NNMT protein levels in WAT of ob/ob mice (n =8) and lean controls (n =4) (c); db/db mice and lean controls (n =7 per group) (d), and high-fat diet (HFD)-fed (n =6) and chow-fed mice (n =7) (e). f–h, NNMT protein levels in liver of ob/ob mice (n =9) and lean controls (n =6) (f); db/db mice and lean controls (n =7 per group) (g); and HFD-fed and chow-fed mice (n =6 per group) (h). Actin was used as a control for western blot analysis and the levels were not different between lean and obese mice. AU, arbitrary units. Error bars, ±s.e.m; *P <0.05.
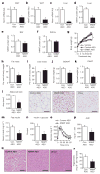
Figure 2. Nnmt knockdown prevents diet-induced obesity and insulin resistance
a–f, Knockdown efficiency of _Nnmt-_ASO. mRNA expression was normalized by cyclophilin and protein levels were corrected with actin levels: Nnmt mRNA (a) and NNMT protein in WAT (b); Nnmt mRNA (c) and NNMT protein in liver (d); NNMT protein in brown adipose tissue (BAT) (e) and kidney (f). g, Body weights of C57BL/6 mice fed a high-fat diet and treated with Nnmt ASO, control ASO, or vehicle (saline) for 8 weeks. h, Fat mass as a percentage of body weight. i, Lean mass as a percentage of body weight. j, Subcutaneous WAT (SQWAT) fat-pad weights. k, Epididymal WAT (EWAT) fat-pad weights. l, Epididymal adipocyte cross-sectional area and haematoxylin and eosin (H&E)-stained sections of SQWAT. m, Serum insulin levels. n, Glucose × insulin product (ng ml−1 × mg dl−1) in the fed state. o, Intraperitoneal glucose tolerance test. p, Area under the curve (AUC) of the glucose tolerance. q, H&E stain of liver sections of HFD-fed _Nnmt_-ASO-and control-ASO-treated mice, and of chow-fed mice. r, Hepatic triglyceride levels in Nnmt- and control-ASO-treated mice. The scale bars in l and q represent 100 μm; n =8 per group for a–p, n =13 per group for r. AU, arbitrary units. Error bars, ±s.e.m.; *P <0.05.
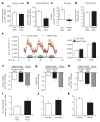
Figure 3. NNMT regulates energy expenditure
a, Energy intake of HFD-fed control-ASO- and _Nnmt_-ASO-treated mice. b, Feed efficiency (body-weight gain per kilocalorie (kcal) consumed) of HFD-fed control-ASO- and _Nnmt–_ASO-treated mice. c, Fat mass gain per kcal consumed in HFD-fed mice treated with Nnmt ASO or control ASO. n =12 per group for a–c. d, Faecal lipid excretion (n =10 per group). e, Oxygen consumption (Vol. O2) measured by CLAMS (n =7 per group). f–h, Effects of a 16-h fast on body weight (f), fat mass (g), and lean body mass (n =8 per group) (h). i–k, Oxygen consumption in 3T3-L1 adipocytes transfected with control or Nnmt ASO (n =6 per group) (i), treated with 10 mM _N_1-methylnicotinamide (me-Nam) (n =6 per group) (j), or transfected with Nnmt cDNA (n =10 per group) (k). Error bars, ±s.e.m.; *P <0.05.
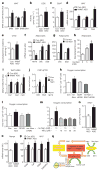
Figure 4. NNMT regulates SAM and NAD+ pathways in adipose tissue
a, Adipose _S_-adenosylmethionine (SAM), _S_-adenosylhomocysteine (SAH) and SAM:SAH ratio measured by LC–MS/MS (n =8 for control-ASO-treated mice; n =12 for _Nnmt_-ASO-treated mice). b, c, ODC (b) and SSAT (c) activity (n =10 per group). d, Amd1, Odc and Ssat mRNA expression in adipose tissue of _Nnmt_-ASO- and control-ASO-treated mice (n =12 per group). e, Urinary diacetylspermine:creatinine ratio (n =22 for control-ASO-treated mice; n =29 for _Nnmt_-ASO-treated mice). f, g, Odc and Ssat mRNA levels in 3T3-L1 adipocytes with Nnmt knockdown (n =9 per group) (f) and N_1-methylnicotinamide (me-Nam) (g) treatment (n =6 per group). h, Diacetylspermine secretion from 3T3-L1 adipocytes treated with me-Nam (n =10 per group). i, Expression of mono-, di- and tri-methylation of lysine histone 3 (H3K4) normalized to total H3 levels in adipose tissue (n =8 per group). j, H3K4me2 occupancy on Odc, Ssat genes and an open reading frame free region (Igx1a) as a negative control in adipocytes measured by ChIP-qPCR (n =12 per group). k–m, Oxygen consumption in adipocytes transfected with control or Ssat siRNA, and treated with or without 10 mM me-Nam (n =6 per group) (k); treated with 10 mM me-Nam with or without 5 mM difluoromethylornithine (DFMO), a specific ODC inhibitor (n =6 per group) (l); or treated with 10 mM me-Nam with or without MDL72527, a specific PAO inhibitor (n =6 per group) (m). n, NAD+ levels (n =7 Con-ASO; n =12 Nnmt ASO). o, mRNA levels of nicotinamide phosphoribosyltransferase (Nampt) (n =11 per group). p, mRNA levels of SIRT1 target genes: Cd36, catalase (Cat), succinate dehydrogenase B (SdhB) and growth arrest and DNA-damage-inducible protein (Gadd45a) in adipose tissue of NNMT-ASO- and control-ASO treated mice (n =11 per group). Error bars, ± s.e.m., *P <0.05, †_P =0.06. q, Model of NNMT-regulated energy expenditure in adipocytes. NNMT methylates nicotinamide (Nam), a precursor of NAD+, using SAM as a methyl donor. SAM regulates polyamine flux by providing substrates and modulating histone methylation. Polyamine flux utilizes acetyl-CoA to generate acetyl-polyamines, which are oxidized or excreted in the urine. Taken together, this results in adipose metabolic substrate consumption and loss coupled with systemic alteration of energy expenditure.
Comment in
- Metabolism: Targeting a fat-accumulation gene.
Brenner C. Brenner C. Nature. 2014 Apr 10;508(7495):194-5. doi: 10.1038/508194a. Nature. 2014. PMID: 24717510 No abstract available.
Similar articles
- Nicotinamide N-methyltransferase: At the crossroads between cellular metabolism and epigenetic regulation.
Roberti A, Fernández AF, Fraga MF. Roberti A, et al. Mol Metab. 2021 Mar;45:101165. doi: 10.1016/j.molmet.2021.101165. Epub 2021 Jan 14. Mol Metab. 2021. PMID: 33453420 Free PMC article. Review. - Glucose availability regulates nicotinamide N-methyltransferase expression in adipocytes.
Ehebauer F, Ghavampour S, Kraus D. Ehebauer F, et al. Life Sci. 2020 May 1;248:117474. doi: 10.1016/j.lfs.2020.117474. Epub 2020 Feb 27. Life Sci. 2020. PMID: 32112869 - Selective and membrane-permeable small molecule inhibitors of nicotinamide N-methyltransferase reverse high fat diet-induced obesity in mice.
Neelakantan H, Vance V, Wetzel MD, Wang HL, McHardy SF, Finnerty CC, Hommel JD, Watowich SJ. Neelakantan H, et al. Biochem Pharmacol. 2018 Jan;147:141-152. doi: 10.1016/j.bcp.2017.11.007. Epub 2017 Nov 15. Biochem Pharmacol. 2018. PMID: 29155147 Free PMC article. - Nicotinamide N-Methyltransferase Interacts with Enzymes of the Methionine Cycle and Regulates Methyl Donor Metabolism.
Hong S, Zhai B, Pissios P. Hong S, et al. Biochemistry. 2018 Oct 9;57(40):5775-5779. doi: 10.1021/acs.biochem.8b00561. Epub 2018 Sep 24. Biochemistry. 2018. PMID: 30226369 - Roles of Nicotinamide N-Methyltransferase in Obesity and Type 2 Diabetes.
Liu JR, Deng ZH, Zhu XJ, Zeng YR, Guan XX, Li JH. Liu JR, et al. Biomed Res Int. 2021 Jul 27;2021:9924314. doi: 10.1155/2021/9924314. eCollection 2021. Biomed Res Int. 2021. PMID: 34368359 Free PMC article. Review.
Cited by
- Knockdown of nicotinamide N-methyltransferase ameliorates renal fibrosis caused by ischemia-reperfusion injury and remodels sphingosine metabolism.
Xu W, Hou L. Xu W, et al. Clin Exp Nephrol. 2024 Dec;28(12):1241-1253. doi: 10.1007/s10157-024-02545-z. Epub 2024 Aug 22. Clin Exp Nephrol. 2024. PMID: 39168882 - Novel tricyclic small molecule inhibitors of Nicotinamide N-methyltransferase for the treatment of metabolic disorders.
Ruf S, Rajagopal S, Kadnur SV, Hallur MS, Rani S, Kristam R, Swaminathan S, Zope BR, Gondrala PK, Swamy I, Putta VPRK, Kandan S, Zech G, Schreuder H, Rudolph C, Elvert R, Czech J, Birudukota S, Siddiqui MA, Anand NN, Mane VS, Dittakavi S, Suresh J, Gosu R, Ramesh M, Yura T, Dhakshinamoorthy S, Kannt A. Ruf S, et al. Sci Rep. 2022 Sep 14;12(1):15440. doi: 10.1038/s41598-022-19634-2. Sci Rep. 2022. PMID: 36104373 Free PMC article. - Novel Inhibitors of Nicotinamide-_N_-Methyltransferase for the Treatment of Metabolic Disorders.
Kannt A, Rajagopal S, Hallur MS, Swamy I, Kristam R, Dhakshinamoorthy S, Czech J, Zech G, Schreuder H, Ruf S. Kannt A, et al. Molecules. 2021 Feb 13;26(4):991. doi: 10.3390/molecules26040991. Molecules. 2021. PMID: 33668468 Free PMC article. - N1-methylnicotinamide impairs gestational glucose tolerance in mice.
Wei X, Tan Y, Huang J, Dong X, Feng W, Liu T, Yang Z, Yang G, Luo X. Wei X, et al. J Mol Endocrinol. 2024 Jan 8;72(2):e230126. doi: 10.1530/JME-23-0126. Print 2024 Feb 1. J Mol Endocrinol. 2024. PMID: 38029302 Free PMC article. - Association of nicotinamide-N-methyltransferase mRNA expression in human adipose tissue and the plasma concentration of its product, 1-methylnicotinamide, with insulin resistance.
Kannt A, Pfenninger A, Teichert L, Tönjes A, Dietrich A, Schön MR, Klöting N, Blüher M. Kannt A, et al. Diabetologia. 2015 Apr;58(4):799-808. doi: 10.1007/s00125-014-3490-7. Epub 2015 Jan 18. Diabetologia. 2015. PMID: 25596852 Free PMC article. Clinical Trial.
References
- Shepherd PR, Kahn BB. Glucose transporters and insulin action—implications for insulin resistance and diabetes mellitus. N Engl J Med. 1999;341:248–257. - PubMed
- Yang Q, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. - PubMed
- Aksoy S, Szumlanski CL, Weinshilboum RM. Human liver nicotinamide N-methyltransferase. cDNA cloning, expression, and biochemical characterization. J Biol Chem. 1994;269:14835–14840. - PubMed
- Riederer M, Erwa W, Zimmermann R, Frank S, Zechner R. Adipose tissue as a source of nicotinamide N-methyltransferase and homocysteine. Atherosclerosis. 2009;204:412–417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK040561/DK/NIDDK NIH HHS/United States
- R37 DK43051/DK/NIDDK NIH HHS/United States
- K08 DK090149/DK/NIDDK NIH HHS/United States
- P01 CA120964/CA/NCI NIH HHS/United States
- P30 DK046200/DK/NIDDK NIH HHS/United States
- 13SDG14620005/AHA_/American Heart Association-American Stroke Association/United States
- R37 DK043051/DK/NIDDK NIH HHS/United States
- P01CA120964/CA/NCI NIH HHS/United States
- T32 DK007516/DK/NIDDK NIH HHS/United States
- R01 DK069966/DK/NIDDK NIH HHS/United States
- P30 CA006516/CA/NCI NIH HHS/United States
- R01 DK100385/DK/NIDDK NIH HHS/United States
- K01 DK094943/DK/NIDDK NIH HHS/United States
- P30CA006516-46/CA/NCI NIH HHS/United States
- R01 DK69966/DK/NIDDK NIH HHS/United States
- P30 DK57521/DK/NIDDK NIH HHS/United States
- P30 DK057521/DK/NIDDK NIH HHS/United States
- R01 DK043051/DK/NIDDK NIH HHS/United States
- P30 DK0460200/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials