Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance - PubMed (original) (raw)
Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance
Louise Hecker et al. Sci Transl Med. 2014.
Abstract
The incidence and prevalence of pathological fibrosis increase with advancing age, although mechanisms for this association are unclear. We assessed the capacity for repair of lung injury in young (2 months) and aged (18 months) mice. Whereas the severity of fibrosis was not different between these groups, aged mice demonstrated an impaired capacity for fibrosis resolution. Persistent fibrosis in lungs of aged mice was characterized by the accumulation of senescent and apoptosis-resistant myofibroblasts. These cellular phenotypes were sustained by alterations in cellular redox homeostasis resulting from elevated expression of the reactive oxygen species-generating enzyme Nox4 [NADPH (reduced form of nicotinamide adenine dinucleotide phosphate) oxidase-4] and an impaired capacity to induce the Nrf2 (NFE2-related factor 2) antioxidant response. Lung tissues from human subjects with idiopathic pulmonary fibrosis (IPF), a progressive and fatal lung disease, also demonstrated this Nox4-Nrf2 imbalance. Nox4 mediated senescence and apoptosis resistance in IPF fibroblasts. Genetic and pharmacological targeting of Nox4 in aged mice with established fibrosis attenuated the senescent, antiapoptotic myofibroblast phenotype and led to a reversal of persistent fibrosis. These studies suggest that loss of cellular redox homeostasis promotes profibrotic myofibroblast phenotypes that result in persistent fibrosis associated with aging. Our studies suggest that restoration of Nox4-Nrf2 redox balance in myofibroblasts may be a therapeutic strategy in age-associated fibrotic disorders, potentially able to resolve persistent fibrosis or even reverse its progression.
Conflict of interest statement
Competing interests: At the time of these studies, E.M. was employed at Genkyotex as the Chief Scientific Officer. However, all authors declare no conflict of interest and there are no financial relationships related to the design and data presented in this manuscript.
Figures
Figure 1
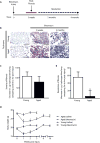
Resolution of fibrosis is impaired in aged mice. C57BL/6 young (2 months) and aged (18 months) mice were subjected to lung injury by airway instillation of intra-tracheal bleomycin (1.25 U/kg). (A) Schematic diagram illustrating the time-course of bleomycin-induced fibrosis and resolution in young mice. (B–D) Lung tissue was harvested at 0 (uninjured), 3, weeks, 2 months, or 4 months post-injury and fibrosis was assessed. Resolution of fibrosis was assessed by Masson’s trichrome blue staining for collagen (B), and whole lung homogenates were analyzed by quantitative hydroxyproline assay (C–D); data are expressed as increase in μg of hydroxyproline/lung comparing control to 3 weeks post-injury (C), or decrease in μg of hydroxyproline/lung comparing 3 weeks to 4 months post-injury (D). Values represent mean ± s.e.m.; n = 5–9 per group; *p value < 0.05, compared with young. (E) Systemic effects in response to bleomycin-induced lung fibrosis or saline control were assessed by a time-course evaluation of body weights. Values represent mean ± s.e.m.; n = 5–20 per group; *p value < 0.05, compared to baseline for each group. Scale bars, 100 μm.
Figure 2
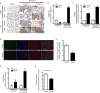
Impaired fibrosis resolution in aged mice is associated with myofibroblast senescence and apoptosis resistance. Young and aged mice were subjected to bleomycin-induced lung fibrosis. Lung tissue was harvested or cells were isolated at various time points following injury. (A) Immunohistochemical analysis of α-SMA expression; myofibroblast marker (upper panels), and p16 expression; senescence marker (lower panels). (B,F) Whole lung tissues were analyzed at the time points indicated by Western blot for protein expression and densitometric analyses of p16 (B) and Bcl-2 (F) was performed; *p value < 0.05, compared with all other groups. (**C**) Fibroblasts from young and aged mice (uninjured and 2 m post-injury) were isolated and cultured _ex vivo_. Senescence was evaluated by quantitative measurement of senescence-associated β-galactosidase (SA-βgal) activity; *_p_ value < 0.05, compared with all other groups. (**D–E**) Lung tissue harvested at 3 weeks post-injury was assessed by immunofluorescence for TUNEL and α-SMA expression (**D**), and apoptotic cells were quantified by counting the number of TUNEL-positive cells/field in >50 random fields of view (E); *p value < 0.05, compared with young. (G) Fibroblasts were isolated from young and aged mice 6 weeks post-injury and cultured ex vivo. Cells were treated with or without staurosporine (300 nM) for 5 h and caspase activity was assessed. Values represent mean ± s.e.m.; n = 3–5; *p value < 0.05, compared with young. Scale bars, 100 μm.
Figure 3
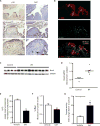
IPF lung myofibroblasts express Nox4 and manifest features of senescence and apoptosis resistance in vitro and in vivo. (A–B) IPF lung tissue sections were analyzed by immunohistochemical analysis (A) and immunofluorescence staining (B). Expression of p16 and Ki67 were evaluated in two different patients (patient 1 in top panels, patient 2 in lower panels). Dashed boxes represent area shown in higher magnification (bottom panels); arrows indicate Ki67+ cells (A). Immunofluorescence labeling for TUNEL, α-SMA, and DAPI in IPF lung tissue sections (B). (C–D) Fibroblasts isolated from the lungs of patients with biopsy-proven IPF (n = 9) and normal-appearing lung tissue from patients undergoing surgical resection for suspected cancer (n=4), and analyzed for protein expression of Nox4 by Western immunoblotting (C); and quantitated by densitometric analysis (D). (E–G) IPF lung fibroblasts were cultured ex vivo and treated with GKT137831 (10 μM) or vehicle (DMSO) for 48 h. H2O2 production was evaluated (E) and senescence was evaluated by quantitative SA-βgal activity assay (F). Cells were treated with or without staurosporine (300 nM) for 8 h and caspase activity was assessed (G). Values represent mean ± s.e.m.; n = 3; *p value < 0.05. Scale bars, 100 μm.
Figure 4
Deficiency in the activation of Nrf2 in fibroblasts from aged mice subjected to lung injury in vivo and in senescent fibroblasts in vitro. (A) IPF lung tissue sections were analyzed for expression of Nrf2 by immunohistochemical analysis. (B–D) Young and aged mice were subjected to bleomycin-induced lung fibrosis. Lung fibroblasts were isolated at the time points indicated and cultured ex vivo. Nrf2 expression was assessed by Western immunoblotting (B) and densitometric analysis (C); *p value < 0.05, compared to aged control. Steady-state H2O2 levels were assessed at the indicated time points by fluorometric assay (D); *p value < 0.05, compared with all other groups. (E–F) Control and senescent IMR90 fibroblasts (at low and high population doublings, respectively) were treated with H2O2 (200 μM). Nrf2 expression was evaluated by Western immunoblotting (E) and quantitated by densitometric analysis (F); *p value < 0.05. Downstream Nrf2-responsive genes were evaluated 16 h post H2O2 treatment by real-time PCR (G); data are expressed as fold increase compared to untreated control (n = 5); *p value < 0.05, compared to non-senescent control. Values represent mean ± s.e.m.; n = 3; *p value < 0.05. Scale bars, 100 μm.
Figure 5
Nox4-Nrf2 imbalance controls redox balance, senescence and apoptosis resistance of fibroblasts. (A–B) Control and senescent cells were evaluated to determine steady-state H2O2 levels (A) or cells were treated with/without staurosporine (300 nM) for 5 h and caspase activity was assessed (B). (C–D) Control and senescent fibroblasts were pre-treated with/without the proteasome inhibitor, MG-132 (25 μM; 2 h), and treated with/without exogenous H2O2 (200 μM) (C) or pre-treated with/without sulforaphane (5 μM; 30 min) (D). Nuclear and cytosolic lysates were evaluated for Nrf2, Lamin and α-tubulin (C–D). (E–F) Fibroblasts were isolated from aged mice at 3 weeks post-injury and cultured ex vivo. Cells were pre-treated with sulforaphane (5 μM) or DMSO for 48 h and Steady-state H2O2 levels were assessed by fluorometric assay (E). Cells were treated with/without staurosporine (300 nM) for 5 h and caspase activity was assessed (F). (G–K) Lung fibroblasts were isolated from Nox4−/− or wild-type mice and cultured ex vivo. Cells were serum starved for 24h, then treated with TGF-β1 (2 ng/ml) for 48 h. (G) H2O2 production was evaluated. (H–K) Cells were treated with or without staurosporine (300 nM) for 8 h and caspase activity was assessed (H). Expression of PARP and caspase-3 were evaluated by western immunoblotting (I) and quantitated by densitometric analysis (J–K). Values represent mean ± s.e.m.; n = 3; *p value < 0.05.
Figure 6
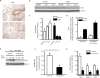
In vivo knockdown of Nox4 restores the capacity for fibrosis resolution in aged mice. (A) Young and aged mice were subjected to bleomycin-induced lung fibrosis. Lung tissues were harvested at 0 (uninjured), 3 weeks, and 2 months post-injury; Nox4 expression was evaluated by immunohistochemical analysis. (B–K) Aged mice subjected to bleomycin-injury were treated with Nox4-targeting or non-targeting siRNA, administered by intranasal delivery every other day for 3 weeks, starting 3 weeks post-injury. A schematic diagram illustrates treatment period following injury (B). (C, F–I) Lung fibroblasts were isolated at the end of treatment (6 weeks post-injury) and cultured ex vivo. Expression of Nox4 (C) p16 (F), p21 (G), Bcl-2 (H), and Col1A1 (I) and were analyzed by Western immunoblotting and quantified by densitometric analyses; values represent mean ± s.e.m.; n = 5–8 per group; *p value < 0.05, compared to NT-siRNA. Fibroblast senescence was evaluated by quantitative measurement of SA-βgal activity (D); n = 3 per group; *p value < 0.05, compared to NT-siRNA; and SA-βgal staining (E). (J–K) Lung tissue was harvested at 6 weeks post-injury; fibrosis was assessed by Masson’s trichrome blue staining for collagen (J), and quantitative hydroxyproline assay (K). Values represent mean ± s.e.m.; n = 6–8 per group; *p value < 0.05, compared to untreated controls. Scale bars, 100 μm.
Figure 7
In vivo pharmacologic targeting of Nox4 with GKT137831 leads to reversal of age-associated persistent fibrosis. (A–D) Aged mice (18 m) were subjected to bleomycin-induced lung fibrosis. Starting at 3 weeks post-injury, mice were treated daily with GKT137831 (40 mg/kg) or vehicle by oral gavage through week 6 (21 treatments total). Body weight of the mice was recorded weekly (A); values represent mean ± s.e.m.; n = 17–21 per group; *p value < 0.05, compared to vehicle-treated controls. (B–C) Lung tissues were harvested from control (uninjured) or at 6 weeks post-injury. Tissues were evaluated by Masson’s trichrome blue staining for collagen and by immunohistochemical analyses was performed to evaluate expression of α-SMA and senescence markers (p16 and p21) (B). Whole lung homogenates were analyzed by quantitative hydroxyproline assay (C); data are expressed as total μg of hydroxyproline/whole lung; values represent mean ± s.e.m.; n = 9–10 per group; *p value < 0.05, compared to all other groups. Kaplan-Meier survival curve for GKT137831 (n = 22) and vehicle (n = 23) treated mice (D); log-rank test, p < 0.05. Scale bars, 100 μm.
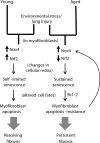
Figure 8
Proposed model for persistent fibrosis in aged mice following lung injury.
Comment in
- Lung disease: resetting the redox balance in lung fibrosis.
Bray N. Bray N. Nat Rev Drug Discov. 2014 Jun;13(6):415. doi: 10.1038/nrd4344. Epub 2014 May 16. Nat Rev Drug Discov. 2014. PMID: 24833297 No abstract available. - Putting the brakes on age-related idiopathic pulmonary fibrosis: can Nox4 inhibitors suppress IPF?
Turn CS, Lockey RF, Kolliputi N. Turn CS, et al. Exp Gerontol. 2015 Mar;63:81-2. doi: 10.1016/j.exger.2015.02.002. Epub 2015 Feb 8. Exp Gerontol. 2015. PMID: 25668226 No abstract available.
Similar articles
- NADPH Oxidases and Aging Models of Lung Fibrosis.
Bernard K, Thannickal VJ. Bernard K, et al. Methods Mol Biol. 2019;1982:487-496. doi: 10.1007/978-1-4939-9424-3_29. Methods Mol Biol. 2019. PMID: 31172491 - Transforming growth factor β1 (TGFβ1)-induced CD44V6-NOX4 signaling in pathogenesis of idiopathic pulmonary fibrosis.
Ghatak S, Hascall VC, Markwald RR, Feghali-Bostwick C, Artlett CM, Gooz M, Bogatkevich GS, Atanelishvili I, Silver RM, Wood J, Thannickal VJ, Misra S. Ghatak S, et al. J Biol Chem. 2017 Jun 23;292(25):10490-10519. doi: 10.1074/jbc.M116.752469. Epub 2017 Apr 7. J Biol Chem. 2017. PMID: 28389561 Free PMC article. - Bromodomain and Extraterminal (BET) Protein Inhibition Restores Redox Balance and Inhibits Myofibroblast Activation.
Stock CJW, Michaeloudes C, Leoni P, Durham AL, Mumby S, Wells AU, Chung KF, Adcock IM, Renzoni EA, Lindahl GE. Stock CJW, et al. Biomed Res Int. 2019 Apr 18;2019:1484736. doi: 10.1155/2019/1484736. eCollection 2019. Biomed Res Int. 2019. PMID: 31119153 Free PMC article. - Redox Imbalance in Idiopathic Pulmonary Fibrosis: A Role for Oxidant Cross-Talk Between NADPH Oxidase Enzymes and Mitochondria.
Veith C, Boots AW, Idris M, van Schooten FJ, van der Vliet A. Veith C, et al. Antioxid Redox Signal. 2019 Nov 10;31(14):1092-1115. doi: 10.1089/ars.2019.7742. Epub 2019 Apr 5. Antioxid Redox Signal. 2019. PMID: 30793932 Free PMC article. Review. - Lung Fibroblasts, Aging, and Idiopathic Pulmonary Fibrosis.
Pardo A, Selman M. Pardo A, et al. Ann Am Thorac Soc. 2016 Dec;13 Suppl 5:S417-S421. doi: 10.1513/AnnalsATS.201605-341AW. Ann Am Thorac Soc. 2016. PMID: 28005427 Review.
Cited by
- Aging Suppresses Sphingosine-1-Phosphate Chaperone ApoM in Circulation Resulting in Maladaptive Organ Repair.
Ding BS, Yang D, Swendeman SL, Christoffersen C, Nielsen LB, Friedman SL, Powell CA, Hla T, Cao Z. Ding BS, et al. Dev Cell. 2020 Jun 22;53(6):677-690.e4. doi: 10.1016/j.devcel.2020.05.024. Epub 2020 Jun 15. Dev Cell. 2020. PMID: 32544390 Free PMC article. - Prognostic model of fibroblasts in idiopathic pulmonary fibrosis by combined bulk and single-cell RNA-sequencing.
Zhao J, Jing C, Fan R, Zhang W. Zhao J, et al. Heliyon. 2024 Jul 11;10(14):e34519. doi: 10.1016/j.heliyon.2024.e34519. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39113997 Free PMC article. - Malic enzyme 1 (ME1) in the biology of cancer: it is not just intermediary metabolism.
Simmen FA, Alhallak I, Simmen RCM. Simmen FA, et al. J Mol Endocrinol. 2020 Nov;65(4):R77-R90. doi: 10.1530/JME-20-0176. J Mol Endocrinol. 2020. PMID: 33064660 Free PMC article. Review. - NOX4 NADPH Oxidase-Dependent Mitochondrial Oxidative Stress in Aging-Associated Cardiovascular Disease.
Vendrov AE, Vendrov KC, Smith A, Yuan J, Sumida A, Robidoux J, Runge MS, Madamanchi NR. Vendrov AE, et al. Antioxid Redox Signal. 2015 Dec 20;23(18):1389-409. doi: 10.1089/ars.2014.6221. Epub 2015 Jul 14. Antioxid Redox Signal. 2015. PMID: 26054376 Free PMC article. - HSP90 Inhibition and Modulation of the Proteome: Therapeutical Implications for Idiopathic Pulmonary Fibrosis (IPF).
Colunga Biancatelli RML, Solopov P, Gregory B, Catravas JD. Colunga Biancatelli RML, et al. Int J Mol Sci. 2020 Jul 25;21(15):5286. doi: 10.3390/ijms21155286. Int J Mol Sci. 2020. PMID: 32722485 Free PMC article. Review.
References
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008 May 15;453:314. - PubMed
- King TE, Jr, et al. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001 Sep 15;164:1025. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- IK2 BX001477/BX/BLRD VA/United States
- R01 HL094230/HL/NHLBI NIH HHS/United States
- P01 HL114470/HL/NHLBI NIH HHS/United States
- P30 AR048311/AR/NIAMS NIH HHS/United States
- P50 HL107181/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases