Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms - PubMed (original) (raw)
Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms
Suryasarathi Dasgupta et al. Cell Host Microbe. 2014.
Abstract
Polysaccharide A (PSA), the archetypical immunomodulatory molecule of the gut commensal Bacteroides fragilis, induces regulatory T cells to secrete the anti-inflammatory cytokine interleukin-10 (IL-10). The cellular mediators of PSA's immunomodulatory properties are incompletely understood. In a mouse model of colitis, we find that PSA requires both innate and adaptive immune mechanisms to generate protection. Plasmacytoid DCs (PDCs) exposed to PSA do not produce proinflammatory cytokines, but instead they specifically stimulate IL-10 secretion by CD4+ T cells and efficiently mediate PSA-afforded immunoprotection. PSA induces and preferentially ligates Toll-like receptor 2 on PDCs but not on conventional DCs. Compared with other TLR2 ligands, PSA is better at enhancing PDC expression of costimulatory molecules required for protection against colitis. PDCs can thus orchestrate the beneficial immunoregulatory interaction of commensal microbial molecules, such as PSA, through both innate and adaptive immune mechanisms.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
Figure 1
PSA-stimulated IL-10 secretion by CD4+ T cells in splenic DC–CD4+ T cell co-culture is dependent on DCs. (A) IL-10 levels in culture supernatants (as measured by ELISA) are significantly higher in wells with PSA than in wells without PSA and suggest DC dependence of IL-10 secretion by CD4+ T-cells. Data represent the average of 4 independent experiments analyzed by Student's _t_-test. ***p<0.001; n.d., not detected; ns, not significant. (B) In co-cultures with IL-10−/− CD4+ T cells and WT DCs but not with WT CD4+ T cells and IL-10−/− DCs, essentially no IL-10 is produced; thus the major source of IL-10 in these co-cultures is CD4+ T cells. n.d., not detected.
Figure 2
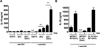
PDCs exhibit a protective phenotype in TNBS-induced colitis after PSA pretreatment. (A) Mice treated with PSA orally (100 μg/dose, TNBS+PSA) before intrarectal challenge with TNBS in a colitis model had a significantly greater (8.52-fold) increase in PDC numbers in MLNs than did mice pretreated with PBS (TNBS+PBS). Each dot represents one mouse on day 3 after TNBS or control buffer administration. PDCs were identified by gating of SH+CD11b−B220+CD11c+ cells. (B) Numbers of CDCs (CD11b−B220+ population gated out from CD11c+ population) in MLNs were not significantly changed in disease or in PSA-treated mice. (C) Augmentation of PDC frequency in MLNs after TNBS challenge (% of SH+ PDCs in the CD11b−B220+CD11c+ population) was significantly greater in mice pretreated with oral PSA (50 μg/dose) than in PBS-treated mice. (D) In TNBS colitis, SH+ PDC frequency in MLNs is significantly but inversely correlated with colitis scores. Horizontal bars in scatter plots represent median values. Unpaired Student’s _t_-test: *p<0.05; **p<0.01; ***p<0.001; ns, not significant. See also Figure S1.
Figure 3
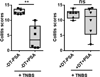
PDC depletion abrogates PSA protection in a TNBS colitis model. PDC depletion by DT administration (200 ng/dose, 6 doses) to BDCA2-DTR mice before inflammation onset inhibits PSA-mediated protection. Boxplots show median (horizontal bar inside box) and quartile distributions in the TNBS model. Left and right panels show colitis scores with and without DT, respectively. Each dot in the boxplots represents an individual mouse. Scores were assessed for statistical significance by two-tailed nonparametric Mann-Whitney test. **p<0.01; ns, not significant. See also Figure S2.
Figure 4
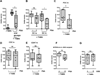
Adoptive transfer of PSA-treated PDCs protects mice in a TNBS colitis model. (A) Protection was seen with oral PSA treatment (100 μg/dose). (B) Colitis scores of WT mice after treatment with CD11c+ BMDCs show protection following adoptive transfer of the higher of two doses of PSA-pretreated, CD11c microbead–selected BMDCs (6–6.5 × 105/dose, 2 doses; PSA-DC hi) but not the lower dose (1–1.5 × 105/dose, 2 doses; PSA-DC lo) when results were compared to those with the respective PBS-pretreated BMDCs. (C) Protection was seen with adoptively transferred lower-dose PSA-pretreated PDCs when results were compared to those with PBS-pretreated PDCs. (D) No protection was seen with low-dose PSA-pretreated CDCs when results were compared to those with PBS-pretreated CDCs. (E) PSA-pretreated CD4+ T cells (5 × 105/dose, 2 doses) did not protect mice when results were compared to those with PBS-pretreated CD4+ T-cell controls. (F) After adoptive transfer to IL-10−/− mice, low-dose PDCs from WT mice pretreated with PSA or PBS failed to confer significant protection. (G) Pretreatment of PDCs with a control polysaccharide (type II polysaccharide from group B Streptococcus) failed to protect mice from TNBS-induced colitis after adoptive transfer. Each dot represents one mouse. Scores were assessed for statistical significance by two-tailed nonparametric Mann-Whitney test. *p<0.05; **p<0.01; ns, not significant. See also Figure S3.
Figure 5
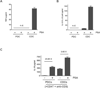
PSA differentially affects diverse DC subsets. (A, B) Levels of proinflammatory cytokines TNF-α (A) and IL-12/IL-23 (B) were measured in supernatants of monocultures containing PDCs or CDCs (5 × 104 cells/ml) that were incubated for 24 h with or without PSA (100 μg/ml). Data represent 2 experiments. (C) IL-10 liberation from CD4+ T cells co-cultured with CDCs and PDCs. IL-10 levels were measured by ELISA of culture supernatants of PDCs or CDCs co-cultured with CD4+ T cells for 5 days in the presence of anti-CD3. Co-cultures were either treated or not treated with PSA (50 μg/ml). Horizontal bars show the fold increase in mean IL-10 production in PSA-treated co-cultures. Data represent the average of 7 independent experiments. Error bars indicate SEM values. See also Figure S6.
Figure 6
PDCs have an immunoregulatory phenotype that is TLR2-dependent. (A) The presence of TLR2 on PDCs (but not on CDCs) augments PSA-generated IL-10 production by CD4+ T cells, as measured by ELISA (average of 3 experiments). (B, C) Elevated frequency of TLR2+ PDCs (SH+PDCA-1+CD11c+) (B), but not of TLR2+ CDCs (SH−CD11c+) (C), after PSA treatment (60 μg/ml). Pam3CSK4 (PAM, 0.1 μg/ml) and FSL-1 (0.1 μg/ml)—but not lipomannan (LM-MS, 10 ng/ml)—augment both TLR2+ PDCs and TLR2+ CDCs. Data are from 2 independent experiments. (D) PDCs treated with PSA but not with other TLR2 ligands show a significant increase (as measured by ELISA) in fold-induction of IL-10 production by CD4+ T cells over that seen with PSA-treated CDCs (average of 3 independent experiments). Error bars in A–D indicate SEM values. (E, F) PSA-induced diminution of colonic lamina propria CD11b+CD11c− cells (E) and augmentation of GMFI of SH in MLNs (F) in TNBS-induced colitis in WT mice but not TLR2−/− mice a similar situation. Unpaired Student’s _t_-test: *p<0.05; **p<0.01; ns, not significant. Error bars indicate SEM values. See also Figure S4.
Figure 7
Cognate interactions are essential for PSA-mediated generation of IL-10 in vitro and for protection in the TNBS colitis model. (A) WT CD4+ T cells co-cultured with PDCs from WT and MHCII−/− mice were compared. Co-cultures with WT PDCs had significantly higher levels of IL-10 than those with MHCII-deficient PDCs. (B) Adoptive transfer of PSA-pretreated BMPDCs from MHCII−/− mice (1.5 × 105/dose, 2 doses) did not confer significant protection to WT mice. (C) Bone marrow–derived PDCs (SH+PDCA-1+CD11c+; gating used for evaluation) included a significantly higher frequency of ICOSL+SH+ cells when treated with PSA (60 μg/ml) than when treated with other TLR2 ligands [Pam3CSK4 (PAM), 0.1 μg/ml; FSL-1, 0.1 μg/ml; and lipomannan (LM-MS), 10 ng/ml] or left untreated (medium control). Bar graph shows average of 5 independent experiments. (D, E) Co-cultures of WT CD4+ T cells with PDCs from ICOSL−/− mice (D) or CD86−/− mice (E) produced significantly less IL-10 than co-cultures of WT CD4+ cells with WT PDCs. (F) Enhanced ability of PDCs from WT mice to stimulate IL-10 production more strongly in WT CD4+ T cells than in CD4+ T cells from ICOS−/− or CD28−/− mice. Data are the average of 2 independent experiments. (G) Adoptive transfer of PSA-pretreated WT PDCs did not confer protection in ICOS−/− recipients. (H) Anti-CD86 IgG and anti-ICOSL IgG blocked PSA-mediated protection and significantly reduced protection from that in IgG isotype control–treated mice. (I) WT mice—but not ICOSL−/− mice—were significantly protected after oral PSA treatment. Data shown in A, C, and D–F were analyzed by unpaired Student's t-test. *, **, and *** denote p<0.05, p<0.01, and p<0.001, respectively. Clinical scores shown in B, G, H, and I were assessed for statistical significance by two-tailed nonparametric Mann-Whitney test. *p<0.05; **p<0.01; ns, not significant. Each dot represents one mouse. Error bars indicate SEM values. See also Figure S5.
Comment in
- Polysaccharide A of Bacteroides fragilis: actions on dendritic cells and T cells.
Kayama H, Takeda K. Kayama H, et al. Mol Cell. 2014 Apr 24;54(2):206-7. doi: 10.1016/j.molcel.2014.04.002. Mol Cell. 2014. PMID: 24766882
Similar articles
- Outer membrane vesicles of a human commensal mediate immune regulation and disease protection.
Shen Y, Giardino Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Shen Y, et al. Cell Host Microbe. 2012 Oct 18;12(4):509-20. doi: 10.1016/j.chom.2012.08.004. Epub 2012 Sep 20. Cell Host Microbe. 2012. PMID: 22999859 Free PMC article. - Polysaccharide A of Bacteroides fragilis: actions on dendritic cells and T cells.
Kayama H, Takeda K. Kayama H, et al. Mol Cell. 2014 Apr 24;54(2):206-7. doi: 10.1016/j.molcel.2014.04.002. Mol Cell. 2014. PMID: 24766882 - A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease.
Ochoa-Repáraz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. Ochoa-Repáraz J, et al. Mucosal Immunol. 2010 Sep;3(5):487-95. doi: 10.1038/mi.2010.29. Epub 2010 Jun 9. Mucosal Immunol. 2010. PMID: 20531465 - Exploring the Gut-Brain Axis for the Control of CNS Inflammatory Demyelination: Immunomodulation by Bacteroides fragilis' Polysaccharide A.
Erturk-Hasdemir D, Ochoa-Repáraz J, Kasper DL, Kasper LH. Erturk-Hasdemir D, et al. Front Immunol. 2021 May 5;12:662807. doi: 10.3389/fimmu.2021.662807. eCollection 2021. Front Immunol. 2021. PMID: 34025663 Free PMC article. Review. - The Intestinal Commensal, Bacteroides fragilis, Modulates Host Responses to Viral Infection and Therapy: Lessons for Exploration during Mycobacterium tuberculosis Infection.
Eribo OA, du Plessis N, Chegou NN. Eribo OA, et al. Infect Immun. 2022 Jan 25;90(1):e0032121. doi: 10.1128/IAI.00321-21. Epub 2021 Oct 4. Infect Immun. 2022. PMID: 34606367 Free PMC article. Review.
Cited by
- Efficacy and safety of Bacteroides fragilis BF839 for pediatric autism spectrum disorder: a randomized clinical trial.
Lin CH, Zeng T, Lu CW, Li DY, Liu YY, Li BM, Chen SQ, Deng YH. Lin CH, et al. Front Nutr. 2024 Sep 3;11:1447059. doi: 10.3389/fnut.2024.1447059. eCollection 2024. Front Nutr. 2024. PMID: 39290561 Free PMC article. - Association Between Gut and Nasal Microbiota and Allergic Rhinitis: A Systematic Review.
Hu Y, Zhang R, Li J, Wang H, Wang M, Ren Q, Fang Y, Tian L. Hu Y, et al. J Asthma Allergy. 2024 Jul 9;17:633-651. doi: 10.2147/JAA.S472632. eCollection 2024. J Asthma Allergy. 2024. PMID: 39006241 Free PMC article. Review. - Neuroactive Steroids, Toll-like Receptors, and Neuroimmune Regulation: Insights into Their Impact on Neuropsychiatric Disorders.
Balan I, Boero G, Chéry SL, McFarland MH, Lopez AG, Morrow AL. Balan I, et al. Life (Basel). 2024 Apr 30;14(5):582. doi: 10.3390/life14050582. Life (Basel). 2024. PMID: 38792602 Free PMC article. Review. - The role of plasmacytoid dendritic cells (pDCs) in immunity during viral infections and beyond.
Ngo C, Garrec C, Tomasello E, Dalod M. Ngo C, et al. Cell Mol Immunol. 2024 Sep;21(9):1008-1035. doi: 10.1038/s41423-024-01167-5. Epub 2024 May 22. Cell Mol Immunol. 2024. PMID: 38777879 Free PMC article. Review. - Critical role of the gut microbiota in immune responses and cancer immunotherapy.
Li Z, Xiong W, Liang Z, Wang J, Zeng Z, Kołat D, Li X, Zhou D, Xu X, Zhao L. Li Z, et al. J Hematol Oncol. 2024 May 14;17(1):33. doi: 10.1186/s13045-024-01541-w. J Hematol Oncol. 2024. PMID: 38745196 Free PMC article. Review.
References
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. - PubMed
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK043351/DK/NIDDK NIH HHS/United States
- R01 DK068181/DK/NIDDK NIH HHS/United States
- R21 AI090102/AI/NIAID NIH HHS/United States
- AI1090102/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous