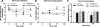β2- and β3-adrenergic receptors drive COMT-dependent pain by increasing production of nitric oxide and cytokines - PubMed (original) (raw)
β2- and β3-adrenergic receptors drive COMT-dependent pain by increasing production of nitric oxide and cytokines
Jane E Hartung et al. Pain. 2014 Jul.
Abstract
Decreased activity of catechol-O-methyltransferase (COMT), an enzyme that metabolizes catecholamines, contributes to pain in humans and animals. Previously, we demonstrated that development of COMT-dependent pain is mediated by both β2- and β3-adrenergic receptors (β2ARs and β3ARs). Here we investigated molecules downstream of β2- and β3ARs driving pain in animals with decreased COMT activity. Based on evidence linking their role in pain and synthesis downstream of β2- and β3AR stimulation, we hypothesized that nitric oxide (NO) and proinflammatory cytokines drive COMT-dependent pain. To test this, we measured plasma NO derivatives and cytokines in rats receiving the COMT inhibitor OR486 in the presence or absence of the β2AR antagonist ICI118,551+β3AR antagonist SR59320A. We also assessed whether the NO synthase inhibitor L-N(G)-nitroarginine methyl ester (L-NAME) and cytokine-neutralizing antibodies block the development of COMT-dependent pain. Results showed that animals receiving OR486 exhibited higher levels of NO derivatives, tumor necrosis factor α (TNFα), interleukin-1β (IL-1β), interleukin-6 (IL-6), and chemokine (C-C motif) ligand 2 (CCL2) in a β2- and β3AR-dependent manner. Additionally, inhibition of NO synthases and neutralization of the innate immunity cytokines TNFα, IL-1β, and IL-6 blocked the development of COMT-dependent pain. Finally, we found that NO influences TNFα, IL-1β, IL-6, and CCL2 levels, whereas TNFα and IL-6 influence NO levels. Altogether, these results demonstrate that β2- and β3ARs contribute to COMT-dependent pain, at least partly, by increasing NO and cytokines. Furthermore, they identify β2- and β3ARs, NO, and proinflammatory cytokines as potential therapeutic targets for pain patients with abnormalities in COMT physiology.
Keywords: Allodynia; Catecholamines; Chemokine (C-C motif) ligand 2 (CCL2); Epinephrine; Hyperalgesia; Inflammation; Interleukin 1 beta (IL-1β); Interleukin 6 (IL-6); Monocyte chemotactic protein 1 (MCP-1); Nitrate; Nitrite; Norepinephrine; Tumor necrosis factor alpha (TNFα).
Copyright © 2014 International Association for the Study of Pain. Published by Elsevier B.V. All rights reserved.
Figures
Fig. 1. Timeline of administered treatments used in this study
The COMT inhibitor OR486 or vehicle was administered in the presence or absence of the β2- and β3-adrenergic receptor antagonists ICI118,551 and SR59320A, the NO synthase inhibitor L-NAME, or neutralizing antibodies against TNFα, IL-1β , IL-6, or CCL2.
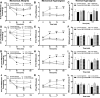
Fig. 2. COMT inhibition increases pain, NO derivatives, and cytokines via β2,3ARs
Animals receiving OR486 (30 mg/kg) exhibit (A) mechanical allodynia, (B) mechanical hyperalgesia, and (C) thermal hyperalgesia, as well as increased circulating levels of (D) nitrite, (E) TNFα, (F) IL-1β, (G) IL-6, and (H) CCL2. COMT-dependent increases in pain, nitrite, and cytokines were completely blocked by co-administration of ICI118,551 (0.5 mg/kg) and SR59320A (5.0mg/kg). N=6-10 per group. Data are mean ± SEM. *P<0.05, **P<0.01, ***P<0.001 different from Veh/Veh, #P<0.05 different ICI+SR/Veh and ICI+SR/OR486.
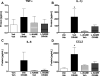
Fig. 3. Inhibition of NO synthesis prevents COMT-dependent pain
Administration of the universal nitric oxide synthase inhibitor L-NAME (30 mg/kg) prior to OR486 (30 mg/kg) normalized (A) mechanical allodynia, (B) mechanical hyperalgesia, and (C) thermal hyperalgesia. N=8-10 per group. Data are mean ± SEM. *P<0.05, **P<0.01, ***P<0.001 different from Veh/Veh.
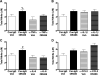
Fig. 4. Neutralization of TNFα, IL-1β, and IL-6, but not CCL2, blocks COMT-dependent pain
Administration of α-TNFα (75 μg), α-IL-1β (75 μg), or α-IL-6 (75 μg) prior to OR486 (30 mg/kg) normalized (A, D, G) mechanical allodynia, (B, E, H) mechanical hyperalgesia, and (C, F, I) thermal hyperalgesia. (J-L) Administration of α-CCL2 failed to block OR486-induced increases in mechanical and thermal pain. N=6-8 per group. Data are mean ± SEM. *P<0.05, **P<0.01, ***P<0.001 different from Control IgG/Veh. #P<0.05 different from α-TNFα/Veh and α-TNFα/OR486.
Fig. 5. Inhibition of NO synthesis prevents COMT-dependent increases in cytokines
Administration of the nitric oxide synthase inhibitor L-NAME (30 mg/kg) prior to OR486 (30 mg/kg) blocked increases in circulating levels of (A) TNFα, (B) IL-1β, (C) IL-6, and (D) CCL2. N=6-10 per group. *P<0.05 different from Veh/Veh.
Fig. 6. Neutralization of TNFα and IL-6 prevents COMT- dependent increases in NO
OR486-induced increases in total nitrite (nitrite and nitrate) were blocked by pretreatment with (A) α-TNFα (75 μg) or (C) α-IL-6 (75 μg), but not (B) α-IL-1β (75 μg) or (D) α-CCL2 (75 μg). N=6-8 per group. Data are mean ± SEM. %P<0.05 different from α-TNFα/Veh, #P< 0.05 different from α-IL-6/Veh and α-IL-6/OR486.
Similar articles
- Sustained stimulation of β2- and β3-adrenergic receptors leads to persistent functional pain and neuroinflammation.
Zhang X, Hartung JE, Bortsov AV, Kim S, O'Buckley SC, Kozlowski J, Nackley AG. Zhang X, et al. Brain Behav Immun. 2018 Oct;73:520-532. doi: 10.1016/j.bbi.2018.06.017. Epub 2018 Jun 20. Brain Behav Immun. 2018. PMID: 29935309 Free PMC article. - Persistent Catechol-O-methyltransferase-dependent Pain Is Initiated by Peripheral β-Adrenergic Receptors.
Ciszek BP, O'Buckley SC, Nackley AG. Ciszek BP, et al. Anesthesiology. 2016 May;124(5):1122-35. doi: 10.1097/ALN.0000000000001070. Anesthesiology. 2016. PMID: 26950706 Free PMC article. - Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both beta2- and beta3-adrenergic receptors.
Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, Maixner W. Nackley AG, et al. Pain. 2007 Apr;128(3):199-208. doi: 10.1016/j.pain.2006.09.022. Epub 2006 Nov 7. Pain. 2007. PMID: 17084978 Free PMC article. - Targeting β3-Adrenergic Receptors in the Heart: Selective Agonism and β-Blockade.
Cannavo A, Koch WJ. Cannavo A, et al. J Cardiovasc Pharmacol. 2017 Feb;69(2):71-78. doi: 10.1097/FJC.0000000000000444. J Cardiovasc Pharmacol. 2017. PMID: 28170359 Free PMC article. Review. - Involvement of cytokines in slow wave sleep.
Krueger JM, Clinton JM, Winters BD, Zielinski MR, Taishi P, Jewett KA, Davis CJ. Krueger JM, et al. Prog Brain Res. 2011;193:39-47. doi: 10.1016/B978-0-444-53839-0.00003-X. Prog Brain Res. 2011. PMID: 21854954 Free PMC article. Review.
Cited by
- Biopsychosocial influence on shoulder pain: Rationale and protocol for a pre-clinical trial.
George SZ, Staud R, Borsa PA, Wu SS, Wallace MR, Greenfield WH, Mackie LN, Fillingim RB. George SZ, et al. Contemp Clin Trials. 2017 May;56:9-17. doi: 10.1016/j.cct.2017.03.005. Epub 2017 Mar 14. Contemp Clin Trials. 2017. PMID: 28315479 Free PMC article. Clinical Trial. - Genetics of ischemic stroke functional outcome.
Carnwath TP, Demel SL, Prestigiacomo CJ. Carnwath TP, et al. J Neurol. 2024 May;271(5):2345-2369. doi: 10.1007/s00415-024-12263-x. Epub 2024 Mar 19. J Neurol. 2024. PMID: 38502340 Free PMC article. Review. - JNK in spinal cord facilitates bone cancer pain in rats through modulation of CXCL1.
Wang ZL, Du TT, Zhang RG. Wang ZL, et al. J Huazhong Univ Sci Technolog Med Sci. 2016 Feb;36(1):88-94. doi: 10.1007/s11596-016-1547-1. Epub 2016 Feb 3. J Huazhong Univ Sci Technolog Med Sci. 2016. PMID: 26838746 - Neuroinflammation and Central Sensitization in Chronic and Widespread Pain.
Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Ji RR, et al. Anesthesiology. 2018 Aug;129(2):343-366. doi: 10.1097/ALN.0000000000002130. Anesthesiology. 2018. PMID: 29462012 Free PMC article. Review. - Genetic and epigenetic regulation of Catechol-O-methyltransferase in relation to inflammation in chronic fatigue syndrome and Fibromyalgia.
Polli A, Hendrix J, Ickmans K, Bakusic J, Ghosh M, Monteyne D, Velkeniers B, Bekaert B, Nijs J, Godderis L. Polli A, et al. J Transl Med. 2022 Oct 25;20(1):487. doi: 10.1186/s12967-022-03662-7. J Transl Med. 2022. PMID: 36284330 Free PMC article.
References
- Akimoto Y, Horinouchi T, Shibano M, Matsushita M, Yamashita Y, Okamoto T, Yamaki F, Tanaka Y, Koike K. Nitric oxide (NO) primarily accounts for endothelium dependent component of B-adrenoceptor-activated smooth muscle relaxation of mouse aorta in response to isoprenaline. J Smooth Muscle Res. 2002;38(4,5):87–99. - PubMed
- Boehning D, Snyder SH. Novel neural modulators. Annu Rev Neurosci. 2003;26:105–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous