Human α-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus - PubMed (original) (raw)
Human α-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus
Gregory T Spear et al. J Infect Dis. 2014.
Abstract
Lactobacillus colonization of the lower female genital tract provides protection from the acquisition of sexually transmitted diseases, including human immunodeficiency virus, and from adverse pregnancy outcomes. While glycogen in vaginal epithelium is thought to support Lactobacillus colonization in vivo, many Lactobacillus isolates cannot utilize glycogen in vitro. This study investigated how glycogen could be utilized by vaginal lactobacilli in the genital tract. Several Lactobacillus isolates were confirmed to not grow in glycogen, but did grow in glycogen-breakdown products, including maltose, maltotriose, maltopentaose, maltodextrins, and glycogen treated with salivary α-amylase. A temperature-dependent glycogen-degrading activity was detected in genital fluids that correlated with levels of α-amylase. Treatment of glycogen with genital fluids resulted in production of maltose, maltotriose, and maltotetraose, the major products of α-amylase digestion. These studies show that human α-amylase is present in the female lower genital tract and elucidates how epithelial glycogen can support Lactobacillus colonization in the genital tract.
Keywords: Lactobacillus; female genital tract; glycogen; maltose; α-amylase.
© The Author 2014. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
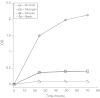
Figure 1.
Lack of L. gasseri growth in glycogen. L. gasseri was cultured in MRS medium that contained either no added carbohydrates (No Carb), 2% glucose, 2% glycogen, or 2% starch for 72 hours. The OD at 600 was read each day. Each culture condition was run in duplicate and the duplicates averaged. Each culture experiment was performed at least 3 times with similar results. Abbreviations: MRS, de Man, Rogosa, and Sharpe; OD, optical density.
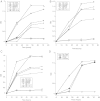
Figure 2.
Growth of lactobacilli in glucose polymers. L. gasseri (A), L. jensenii (B and D), and L. johnsonii (C) were cultured in MRS medium that contained either no added carbohydrates (No Carb) or 2% of either glucose, maltose, maltodextrin 4–7, maltodextrin 13–17, maltodextrin 16–19, maltotriose, or maltopentaose for 72 hours. The OD at 600 was read each day. Abbreviations: MD, maltodextrin; MRS, de Man, Rogosa, and Sharpe; OD, optical density.
Figure 3.
Growth of lactobacilli in salivary α-amylase-treated glycogen. L. gasseri (A), L. jensenii (B), or L. johnsonii (C) were cultured in MRS broth that contained either no added carbohydrates (No Carb), 2% glucose, 2% glycogen, 2.5% saliva alone, or glycogen plus saliva for 72 hours. The OD at 600 was read each day. Abbreviations: MRS, de Man, Rogosa, and Sharpe; OD, optical density.
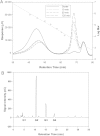
Figure 4.
Degradation of glycogen by salivary α-amylase. Glycogen (2% oyster) was incubated for 10 minutes with no saliva (Oyster) or for 10, 30, or 120 minutes with 2.5% saliva. The size of the degraded glycogen was determined by HPSEC (A) and the products analyzed by HPAEC (B). Abbreviations: HPAEC, high-performance anion-exchange chromatography; HPSEC, high-pressure size exclusion chromatography.
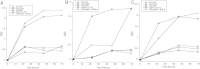
Figure 5.
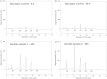
Temperature-dependent degradation of glycogen in genital fluid. A, Aliquots of genital fluid collected from 2 subjects (A and B) were incubated at either 37°C or 4°C for 90 minutes and the level of endogenous glycogen was measured with a colorimetric iodine assay. B, Glycogen was added to genital fluids collected from 4 other women (C–F) or saline, and aliquots were incubated at either 4°C or 37°C for 90 minutes. At the end of the incubation, the amount of glycogen in the samples was measured with the colorimetric iodine assay. C, Degradation in 21 genital fluid samples was measured as in B and the percent degradation was calculated by the following formula: 1 – glycogen 37/glycogen 4 × 100.
Figure 6.
Relationship of glycogen degradation with genital bacteria, vaginal pH, α-amylase and α-glucosidase. The percent of glycogen degradation (Figure 5_C_) in 21 samples was plotted along with either genital-tract levels of L. iners (A), L. crispatus (B), G. vaginalis (C), vaginal pH (D), pancreatic α-amylase (E), or α-glucosidase (F).
Figure 7.
Breakdown of glycogen by genital fluids. Glycogen was incubated for 0 hours (A) or 48 hours (B) with saline or 48 hours with genital fluid collected in saline from 2 different subjects (C and D). After incubation the size of small carbohydrates was determined by HPAEC. Abbreviation: HPAEC, high-performance anion-exchange chromatography.
Similar articles
- Amylases in the Human Vagina.
Nunn KL, Clair GC, Adkins JN, Engbrecht K, Fillmore T, Forney LJ. Nunn KL, et al. mSphere. 2020 Dec 9;5(6):e00943-20. doi: 10.1128/mSphere.00943-20. mSphere. 2020. PMID: 33298571 Free PMC article. - Effect of pH on Cleavage of Glycogen by Vaginal Enzymes.
Spear GT, McKenna M, Landay AL, Makinde H, Hamaker B, French AL, Lee BH. Spear GT, et al. PLoS One. 2015 Jul 14;10(7):e0132646. doi: 10.1371/journal.pone.0132646. eCollection 2015. PLoS One. 2015. PMID: 26171967 Free PMC article. - Glycogen-Degrading Activities of Catalytic Domains of α-Amylase and α-Amylase-Pullulanase Enzymes Conserved in Gardnerella spp. from the Vaginal Microbiome.
Bhandari P, Tingley J, Abbott DW, Hill JE. Bhandari P, et al. J Bacteriol. 2023 Feb 22;205(2):e0039322. doi: 10.1128/jb.00393-22. Epub 2023 Feb 6. J Bacteriol. 2023. PMID: 36744900 Free PMC article. - Unraveling the Dynamics of the Human Vaginal Microbiome.
Nunn KL, Forney LJ. Nunn KL, et al. Yale J Biol Med. 2016 Sep 30;89(3):331-337. eCollection 2016 Sep. Yale J Biol Med. 2016. PMID: 27698617 Free PMC article. Review. - Intrinsic and extrinsic carbohydrates in the vagina: A short review on vaginal glycogen.
Tester R, Al-Ghazzewi FH. Tester R, et al. Int J Biol Macromol. 2018 Jun;112:203-206. doi: 10.1016/j.ijbiomac.2018.01.166. Epub 2018 Jan 31. Int J Biol Macromol. 2018. PMID: 29391223 Review.
Cited by
- Urogenital colonization and pathogenicity of E. Coli in the vaginal microbiota during pregnancy.
Boutouchent N, Vu TNA, Landraud L, Kennedy SP. Boutouchent N, et al. Sci Rep. 2024 Oct 26;14(1):25523. doi: 10.1038/s41598-024-76438-2. Sci Rep. 2024. PMID: 39462143 Free PMC article. - Effects of Dietary Quality on Vaginal Microbiome Composition Throughout Pregnancy in a Multi-Ethnic Cohort.
Miller C, Morikawa K, Benny P, Riel J, Fialkowski MK, Qin Y, Khadka V, Lee MJ. Miller C, et al. Nutrients. 2024 Oct 8;16(19):3405. doi: 10.3390/nu16193405. Nutrients. 2024. PMID: 39408372 Free PMC article. - The cervicovaginal metabolome in women with favorable induction cervix and those unfavorable for induction when delivering at term.
Chen P, Hu T, Zheng Z, Garfield RE, Yang J. Chen P, et al. Heliyon. 2024 Jul 6;10(13):e34166. doi: 10.1016/j.heliyon.2024.e34166. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39071700 Free PMC article. - Updates and Current Challenges in Reproductive Microbiome: A Comparative Analysis between Cows and Women.
Zangirolamo AF, Souza AK, Yokomizo DN, Miguel AKA, Costa MCD, Alfieri AA, Seneda MM. Zangirolamo AF, et al. Animals (Basel). 2024 Jul 3;14(13):1971. doi: 10.3390/ani14131971. Animals (Basel). 2024. PMID: 38998083 Free PMC article. Review. - The dynamics of the female microbiome: unveiling abrupt changes of microbial domains across body sites from prepartum to postpartum phases.
Neumann CJ, Pausan M-R, Haid V, Weiss E-C, Kolovetsiou-Kreiner V, Amtmann B, Winkler P, Mahnert A, Jantscher-Krenn E, Moissl-Eichinger C. Neumann CJ, et al. Microbiol Spectr. 2024 Jun 25;12(8):e0014724. doi: 10.1128/spectrum.00147-24. Online ahead of print. Microbiol Spectr. 2024. PMID: 38917430 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources