Force generation by endocytic actin patches in budding yeast - PubMed (original) (raw)
Force generation by endocytic actin patches in budding yeast
Anders E Carlsson et al. Biophys J. 2014.
Abstract
Membrane deformation during endocytosis in yeast is driven by local, templated assembly of a sequence of proteins including polymerized actin and curvature-generating coat proteins such as clathrin. Actin polymerization is required for successful endocytosis, but it is not known by what mechanisms actin polymerization generates the required pulling forces. To address this issue, we develop a simulation method in which the actin network at the protein patch is modeled as an active gel. The deformation of the gel is treated using a finite-element approach. We explore the effects and interplay of three different types of force driving invagination: 1), forces perpendicular to the membrane, generated by differences between actin polymerization rates at the edge of the patch and those at the center; 2), the inherent curvature of the coat-protein layer; and 3), forces parallel to the membrane that buckle the coat protein layer, generated by an actomyosin contractile ring. We find that with optimistic estimates for the stall stress of actin gel growth and the shear modulus of the actin gel, actin polymerization can generate almost enough force to overcome the turgor pressure. In combination with the other mechanisms, actin polymerization can the force over the critical value.
Copyright © 2014 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
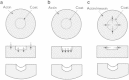
Figure 1
Schematic of proposed force-generation mechanisms for actin-driven endocytosis. Black arrows denote forces exerted by the actin network on coat-proteins/membrane, and gray arrows denote forces exerted by coat proteins/membrane on the actin network. (a) Vertical forces caused by actin polymerization in the outer region or actomyosin contraction in the central region. (b) Spontaneous bending of the coat-protein layer. (c) Horizontal forces resulting from contraction of actomyosin around the coat-protein layer.
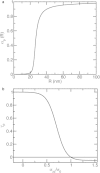
Figure 2
(a) Dependence of the actin polymerization rate factor αp on radial coordinate R. (b) Dependence of the actin polymerization rate factor γp on stress σzz; v∞=−0.05.
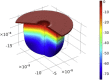
Figure 3
Three-dimensional view of invagination induced by actin polymerization at time t=3 time units. Colors (see scale at right) denote vertical displacement, in units of 10−8m. σ0=104Pa; other parameters have baseline values (Table 1), except that P0=5×104Pa. To see this figure in color, go online.
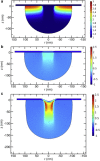
Figure 4
(a) Growth profile in actin gel, after 1 time unit of growth. Colors (see scale at right) denote growth G zZ, which is dimensionless. Parameters: σ0=104Pa, other parameters have baseline values (Table 1), except for P0=5×104Pa. (b) Stress distribution in actin gel after 1 time unit of growth. Stress (colors) is given in units of 105Pa. Parameters are as in a. (c) Same as b, but after 3 time units. To see this figure in color, go online.
Figure 5
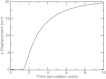
Displacement at the center of the endocytic site as a function of time. Parameters are baseline values, except that P0=5×104Pa and σ0=104Pa.
Figure 6
(a) Indentation distance, Δz, after 5 time units of simulation, as function of actin stall stress, σ0. (b) Pressure at the center of the endocytic site after 5 time units of simulation, as a function of σ0. Parameters are baseline parameters (Table 1) unless otherwise indicated. Alternate symbols show effects of decreasing turgor pressure, P0 (squares), or actin shear modulus, μa (triangles).
Figure 7
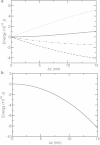
(a) Energy change from coat-protein deformation (dashed line) pushing against turgor pressure (dotted line), and their sum (solid line). Energies are given in 10−18J. Parameters: turgor pressure, P0=2×105Pa, and κc=1.56×10−18J are chosen so that the initial slope of total energy vanishes. Dot-dashed line gives total energy when P0=105Pa. (b) Energy change from myosin contraction, with T=400pN.
Similar articles
- Actin growth profile in clathrin-mediated endocytosis.
Tweten DJ, Bayly PV, Carlsson AE. Tweten DJ, et al. Phys Rev E. 2017 May;95(5-1):052414. doi: 10.1103/PhysRevE.95.052414. Epub 2017 May 23. Phys Rev E. 2017. PMID: 28618637 Free PMC article. - Pulling-force generation by ensembles of polymerizing actin filaments.
Motahari F, Carlsson AE. Motahari F, et al. Phys Biol. 2019 Dec 13;17(1):016005. doi: 10.1088/1478-3975/ab59bd. Phys Biol. 2019. PMID: 31747656 Free PMC article. - Actin assembly produces sufficient forces for endocytosis in yeast.
Nickaeen M, Berro J, Pollard TD, Slepchenko BM. Nickaeen M, et al. Mol Biol Cell. 2019 Jul 22;30(16):2014-2024. doi: 10.1091/mbc.E19-01-0059. Epub 2019 Jun 26. Mol Biol Cell. 2019. PMID: 31242058 Free PMC article. - Local actin polymerization during endocytic carrier formation.
Hinze C, Boucrot E. Hinze C, et al. Biochem Soc Trans. 2018 Jun 19;46(3):565-576. doi: 10.1042/BST20170355. Epub 2018 Apr 20. Biochem Soc Trans. 2018. PMID: 29678956 Review. - Phospho-regulation of intrinsically disordered proteins for actin assembly and endocytosis.
Miao Y, Tipakornsaowapak T, Zheng L, Mu Y, Lewellyn E. Miao Y, et al. FEBS J. 2018 Aug;285(15):2762-2784. doi: 10.1111/febs.14493. Epub 2018 May 17. FEBS J. 2018. PMID: 29722136 Review.
Cited by
- Single-molecule turnover dynamics of actin and membrane coat proteins in clathrin-mediated endocytosis.
Lacy MM, Baddeley D, Berro J. Lacy MM, et al. Elife. 2019 Dec 19;8:e52355. doi: 10.7554/eLife.52355. Elife. 2019. PMID: 31855180 Free PMC article. - Type-I myosins promote actin polymerization to drive membrane bending in endocytosis.
Manenschijn HE, Picco A, Mund M, Rivier-Cordey AS, Ries J, Kaksonen M. Manenschijn HE, et al. Elife. 2019 Aug 6;8:e44215. doi: 10.7554/eLife.44215. Elife. 2019. PMID: 31385806 Free PMC article. - Actin growth profile in clathrin-mediated endocytosis.
Tweten DJ, Bayly PV, Carlsson AE. Tweten DJ, et al. Phys Rev E. 2017 May;95(5-1):052414. doi: 10.1103/PhysRevE.95.052414. Epub 2017 May 23. Phys Rev E. 2017. PMID: 28618637 Free PMC article. - The Process of Wrapping Virus Revealed by a Force Tracing Technique and Simulations.
Pan Y, Zhang F, Zhang L, Liu S, Cai M, Shan Y, Wang X, Wang H, Wang H. Pan Y, et al. Adv Sci (Weinh). 2017 Apr 26;4(9):1600489. doi: 10.1002/advs.201600489. eCollection 2017 Sep. Adv Sci (Weinh). 2017. PMID: 28932658 Free PMC article. - AP3M harbors actin filament binding activity that is crucial for vacuole morphology and stomatal closure in Arabidopsis.
Zheng W, Jiang Y, Wang X, Huang S, Yuan M, Guo Y. Zheng W, et al. Proc Natl Acad Sci U S A. 2019 Sep 3;116(36):18132-18141. doi: 10.1073/pnas.1901431116. Epub 2019 Aug 20. Proc Natl Acad Sci U S A. 2019. PMID: 31431522 Free PMC article.
References
- Wu F., Yao P.J. Clathrin-mediated endocytosis and Alzheimer’s disease: an update. Ageing Res. Rev. 2009;8:147–149. - PubMed
- Kaksonen M., Sun Y., Drubin D.G. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell. 2003;115:475–487. - PubMed
- Kaksonen M., Toret C.P., Drubin D.G. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell. 2005;123:305–320. - PubMed
- Kaksonen M., Toret C.P., Drubin D.G. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2006;7:404–414. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases