Cellular mechanisms of tissue fibrosis. 8. Current and future drug targets in fibrosis: focus on Rho GTPase-regulated gene transcription - PubMed (original) (raw)
Review
Cellular mechanisms of tissue fibrosis. 8. Current and future drug targets in fibrosis: focus on Rho GTPase-regulated gene transcription
Pei-Suen Tsou et al. Am J Physiol Cell Physiol. 2014.
Abstract
Tissue fibrosis occurs with excessive extracellular matrix deposition from myofibroblasts, resulting in tissue scarring and inflammation. It is driven by multiple mediators, such as the G protein-coupled receptor ligands lysophosphatidic acid and endothelin, as well as signaling by transforming growth factor-β, connective tissue growth factor, and integrins. Fibrosis contributes to 45% of deaths in the developed world. As current therapeutic options for tissue fibrosis are limited and organ transplantation is the only effective treatment for end-stage disease, there is an imminent need for efficacious antifibrotic therapies. This review discusses the various molecular pathways involved in fibrosis. It highlights the Rho GTPase signaling pathway and its downstream gene transcription output through myocardin-related transcription factor and serum response factor as a convergence point for targeting this complex set of diseases.
Keywords: Rho GTPase; drug development; fibrosis; gene transcription; therapeutics.
Copyright © 2014 the American Physiological Society.
Figures
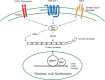
Fig. 1.
Rho GTPase signaling pathway represents a convergent approach to targeting fibrosis. Many current drugs being developed for fibrotic diseases are targeting specific receptors known to be involved in stimulating fibroblasts into myofibroblasts. Interestingly, many of these specific receptor systems converge onto Rho small GTPase signaling. GTP-bound active Rho can interact with downstream effector proteins, most notably Rho-associated, coiled-coil containing protein kinase (ROCK) kinase and mouse diaphanous-related formin-1 (mDia1), which together initiate and stabilize actin stress fibers. This increase in F-actin and resulting decrease in G-actin monomers frees myocardin-related transcription factor (MRTF) to translocate into the nucleus where it cooperates with serum response factor (SRF) to induce gene transcription. Many MRTF/SRF target genes are known drivers of fibrosis including connective tissue growth factor (CTGF), α-smooth muscle actin (α-SMA), and collagen; together this activation of gene transcription induces and maintains the activation of fibroblasts to myofibroblasts. GPCR, G protein-coupled receptor; TGFβ, transforming growth factor-β.
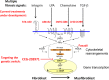
Fig. 2.
Schematic model of current therapeutic approaches. Several receptor mechanisms being targeted in fibrosis are illustrated. All can activate Rho GTPase and the downstream MRTF/SRF gene transcription mechanism. By blocking MRTF-regulated gene transcription and myofibroblasts, our compounds (e.g., CCG-203971) represent a novel approach targeting a key convergence point (genetic switch) in the intrinsic fibroblast-myofibroblast conversion. This may prove more effective than attempting to disrupt each individual profibrotic input. LPA, lysophosphatidic acid; LARG: Leukemia-associated RhoGEF; GEF: guanine nucleotide exchange factor.
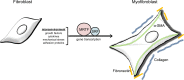
Fig. 3.
The fibroblast-to-myofibroblast transition requires MRTF/SRF gene transcription. α-SMA-expressing myofibroblasts are responsible for the excess deposition of extracellular matrix components observed in pathological fibrosis. Most notably, collagen and fibronectin play important roles in the contractile and fibrous tissue. A variety of microenvironment conditions including growth factors, cytokines, mechanical stress and adhesion proteins combine to propagate the stimulatory response into MRTF/SRF-regulated gene transcription and transition into a myofibroblast state.
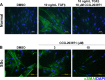
Fig. 4.
CCG-203971 modulates myofibroblast transition of dermal fibroblasts. A: primary human dermal fibroblasts from normal donors were treated with or without 10 ng/ml TGFβ for 3 days to induce a myofibroblast transition; during stimulation, cells were also treated with 10 μM CCG-203971 or DMSO. CCG-203971 markedly decreased α-SMA levels induced by TGFβ. Two representative individual samples are shown. B: elevated α-SMA expression in dermal fibroblasts from patients with diffuse systemic sclerosis (SSc) was blocked by CCG-203971. Two representative individual samples are shown. DAPI, 4′,6-diamidino-2-phenylindole. [From Haak et al. (40). Adapted with permission from the American Society for Pharmacology and Experimental Therapeutics.]
Similar articles
- Novel Rho/MRTF/SRF inhibitors block matrix-stiffness and TGF-β-induced fibrogenesis in human colonic myofibroblasts.
Johnson LA, Rodansky ES, Haak AJ, Larsen SD, Neubig RR, Higgins PD. Johnson LA, et al. Inflamm Bowel Dis. 2014 Jan;20(1):154-65. doi: 10.1097/01.MIB.0000437615.98881.31. Inflamm Bowel Dis. 2014. PMID: 24280883 Free PMC article. - Targeting the myofibroblast genetic switch: inhibitors of myocardin-related transcription factor/serum response factor-regulated gene transcription prevent fibrosis in a murine model of skin injury.
Haak AJ, Tsou PS, Amin MA, Ruth JH, Campbell P, Fox DA, Khanna D, Larsen SD, Neubig RR. Haak AJ, et al. J Pharmacol Exp Ther. 2014 Jun;349(3):480-6. doi: 10.1124/jpet.114.213520. Epub 2014 Apr 4. J Pharmacol Exp Ther. 2014. PMID: 24706986 Free PMC article. - Lysophosphatidic acid signaling through its receptor initiates profibrotic epithelial cell fibroblast communication mediated by epithelial cell derived connective tissue growth factor.
Sakai N, Chun J, Duffield JS, Lagares D, Wada T, Luster AD, Tager AM. Sakai N, et al. Kidney Int. 2017 Mar;91(3):628-641. doi: 10.1016/j.kint.2016.09.030. Epub 2016 Dec 4. Kidney Int. 2017. PMID: 27927603 Free PMC article. - Human Fibrotic Diseases: Current Challenges in Fibrosis Research.
Rosenbloom J, Macarak E, Piera-Velazquez S, Jimenez SA. Rosenbloom J, et al. Methods Mol Biol. 2017;1627:1-23. doi: 10.1007/978-1-4939-7113-8_1. Methods Mol Biol. 2017. PMID: 28836191 Review. - The actin-MRTF-SRF gene regulatory axis and myofibroblast differentiation.
Small EM. Small EM. J Cardiovasc Transl Res. 2012 Dec;5(6):794-804. doi: 10.1007/s12265-012-9397-0. Epub 2012 Aug 17. J Cardiovasc Transl Res. 2012. PMID: 22898751 Review.
Cited by
- Scarring and Skin Fibrosis Reversal with Regenerative Surgery and Stem Cell Therapy.
Almadori A, Butler PE. Almadori A, et al. Cells. 2024 Mar 3;13(5):443. doi: 10.3390/cells13050443. Cells. 2024. PMID: 38474408 Free PMC article. Review. - Therapeutic potential of melatonin in targeting molecular pathways of organ fibrosis.
Hosseinzadeh A, Pourhanifeh MH, Amiri S, Sheibani M, Irilouzadian R, Reiter RJ, Mehrzadi S. Hosseinzadeh A, et al. Pharmacol Rep. 2024 Feb;76(1):25-50. doi: 10.1007/s43440-023-00554-5. Epub 2023 Nov 23. Pharmacol Rep. 2024. PMID: 37995089 Review. - Cellular mechanotransduction in health and diseases: from molecular mechanism to therapeutic targets.
Di X, Gao X, Peng L, Ai J, Jin X, Qi S, Li H, Wang K, Luo D. Di X, et al. Signal Transduct Target Ther. 2023 Jul 31;8(1):282. doi: 10.1038/s41392-023-01501-9. Signal Transduct Target Ther. 2023. PMID: 37518181 Free PMC article. Review. - Relaxin in fibrotic ligament diseases: Its regulatory role and mechanism.
Yuan S, Guo D, Liang X, Zhang L, Zhang Q, Xie D. Yuan S, et al. Front Cell Dev Biol. 2023 Apr 11;11:1131481. doi: 10.3389/fcell.2023.1131481. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37123405 Free PMC article. Review. - 3D-printed long-acting 5-fluorouracil implant to prevent conjunctival fibrosis in glaucoma.
Ioannou N, Luo J, Qin M, Di Luca M, Mathew E, Tagalakis AD, Lamprou DA, Yu-Wai-Man C. Ioannou N, et al. J Pharm Pharmacol. 2023 Feb 8;75(2):276-286. doi: 10.1093/jpp/rgac100. J Pharm Pharmacol. 2023. PMID: 36617180 Free PMC article.
References
- Abu El-Asrar AM, Mohammad G, Nawaz MI, Siddiquei MM, Kangave D, Opdenakker G. Expression of lysophosphatidic acid, autotaxin and acylglycerol kinase as biomarkers in diabetic retinopathy. Acta Diabetol 50: 363–371, 2013 - PubMed
- Akhmetshina A, Dees C, Pileckyte M, Szucs G, Spriewald BM, Zwerina J, Distler O, Schett G, Distler JH. Rho-associated kinases are crucial for myofibroblast differentiation and production of extracellular matrix in scleroderma fibroblasts. Arthritis Rheum 58: 2553–2564, 2008 - PubMed
- Annes JP, Rifkin DB, Munger JS. The integrin αVβ6 binds and activates latent TGFβ3. FEBS Lett 511: 65–68, 2002 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials