Proteomic analysis of intact flagella of procyclic Trypanosoma brucei cells identifies novel flagellar proteins with unique sub-localization and dynamics - PubMed (original) (raw)
. 2014 Jul;13(7):1769-86.
doi: 10.1074/mcp.M113.033357. Epub 2014 Apr 16.
Daria Julkowska 1, Laetitia Vincensini 1, Nele Reeg 1, Johanna Buisson 1, Thierry Blisnick 1, Diego Huet 1, Sylvie Perrot 1, Julien Santi-Rocca 1, Magalie Duchateau 2, Véronique Hourdel 2, Jean-Claude Rousselle 3, Nadège Cayet 4, Abdelkader Namane 3, Julia Chamot-Rooke 2, Philippe Bastin 5
Affiliations
- PMID: 24741115
- PMCID: PMC4083114
- DOI: 10.1074/mcp.M113.033357
Proteomic analysis of intact flagella of procyclic Trypanosoma brucei cells identifies novel flagellar proteins with unique sub-localization and dynamics
Ines Subota et al. Mol Cell Proteomics. 2014 Jul.
Abstract
Cilia and flagella are complex organelles made of hundreds of proteins of highly variable structures and functions. Here we report the purification of intact flagella from the procyclic stage of Trypanosoma brucei using mechanical shearing. Structural preservation was confirmed by transmission electron microscopy that showed that flagella still contained typical elements such as the membrane, the axoneme, the paraflagellar rod, and the intraflagellar transport particles. It also revealed that flagella severed below the basal body, and were not contaminated by other cytoskeletal structures such as the flagellar pocket collar or the adhesion zone filament. Mass spectrometry analysis identified a total of 751 proteins with high confidence, including 88% of known flagellar components. Comparison with the cell debris fraction revealed that more than half of the flagellum markers were enriched in flagella and this enrichment criterion was taken into account to identify 212 proteins not previously reported to be associated to flagella. Nine of these were experimentally validated including a 14-3-3 protein not yet reported to be associated to flagella and eight novel proteins termed FLAM (FLAgellar Member). Remarkably, they localized to five different subdomains of the flagellum. For example, FLAM6 is restricted to the proximal half of the axoneme, no matter its length. In contrast, FLAM8 is progressively accumulating at the distal tip of growing flagella and half of it still needs to be added after cell division. A combination of RNA interference and Fluorescence Recovery After Photobleaching approaches demonstrated very different dynamics from one protein to the other, but also according to the stage of construction and the age of the flagellum. Structural proteins are added to the distal tip of the elongating flagellum and exhibit slow turnover whereas membrane proteins such as the arginine kinase show rapid turnover without a detectible polarity.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Fig. 1.
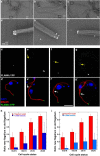
Morphological characterization of purified T. brucei flagella. Flagella were purified from procyclic FLA1RNAi cells grown in the presence of tetracycline for 72h, fixed, and sectioned before analysis by transmission electron microscopy. A, Low magnification view of a preparation of purified flagella. The membrane is preserved in ∼80% of the sectioned flagella. B, Higher magnification of a separate preparation of flagella. Although flagella are highly abundant, small cell debris (containing membrane and subpellicular microtubules) are sometimes encountered (arrow). Flagellar membrane remnants are also present. C, Section through the base of a purified flagellum reveals the typical aspect of the basal body (BB), the transition zone (TZ), the axoneme (AXO). and the emergence of the paraflagellar rod (PFR_). D, E,_ Sections through the basal body area of purified flagella showing the absence of the kinetoplast. F–H, Sections through the transition zone (F), the axoneme (G) or the axoneme and the PFR (H) of purified flagella showed that these structures appear intact. IFT particles can be detected on cross-sections of flagella (white arrows) and occupy the usual position relative to axoneme microtubules. Scale bars, A–B, 2 μm; D–H, 100 nm.
Fig. 2.
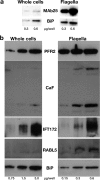
Marker proteins are enriched in flagellar preparations. A, Western blotting analysis of increasing concentrations of total cells or purified flagella (indicated in μg of proteins per well below the figure) probed with the axonemal marker MAb25 or with the endoplasmic reticulum component BiP. A 15-fold enrichment of the MAb25 signal is observed relative to that obtained for BiP. B, Western blotting analysis of increasing concentrations of total cells or a separate preparation of purified flagella (indicated in μg of proteins per lane below the figure) probed with markers of the PFR (PFR2), the membrane (calflagins) or of IFT particles (IFT172, RABL5/IFT22). Flagellar proteins are enriched three- to 15-fold compared with the endoplasmic reticulum marker BiP.
Fig. 3.
Arginine kinase is localized to the flagellum membrane. A–C, IFA of PFA-fixed control trypanosomes stained with an antiserum against the arginine kinase of T. cruzi (white in a, green in B, C), with the PFR marker L8C4 (B, red) and with the axonemal marker MAb25 (C, blue) reveals a membrane location for arginine kinase. D–F, IFA of methanol-fixed trypanosomes stained with the anti-arginine kinase (white in D, green in E, F), with the anti-IFT172 monoclonal antibody (E, red), and with the axonemal marker MAb25 (F, blue). Scale bar, 3 μm. White boxes indicate the area of magnification shown on the right of each panel.
Fig. 4.
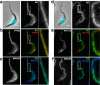
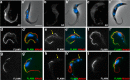
Novel proteins show distinct localizations within the flagellum. A–D, IFA of methanol-fixed trypanosomes expressing the indicated YFP fusion proteins from the endogenous locus stained with an anti-GFP antiserum (green) and with the axonemal marker MAb25 (red) reveals location to the PFR (A, FLAM1), to the PFR region limited to the adhesion domain to the cell body (B, FLAM3), to the proximal axoneme region (C, FLAM6, notice the yellow superposition profile with MAb25) or to the distal end of the flagellum (D, FLAM8). The white arrow in b indicates a partially detached flagellum, showing the association of FLAM3 with the flagellum and not with the attachment zone in the cell body. Scale bar, 5 μm.
Fig. 5.
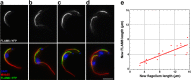
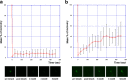
FLAM6 is restricted to the proximal portion of the axoneme at every stage of flagellum formation. A–D, IFA of methanol-fixed trypanosomes expressing the FLAM6::YFP fusion protein stained with an anti-GFP antiserum (green) and with the axonemal marker MAb25 (red) at various stages of flagellum elongation. FLAM6 is restricted to about half of the axoneme at all stages of formation of the new flagellum. E, The length of the FLAM6 signal was measured in 42 cells assembling a new flagellum and plotted versus the actual length of the new flagellum measured from the MAb25 signal. A clear linear relationship is observed, showing that FLAM6 is always restricted to ∼45% of the new flagellum length. Scale bar, 5 μm.
Fig. 6.
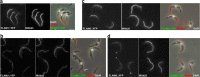
FLAM8 is localized at the tip of axonemal microtubules and accumulates during flagellum construction. A–C, Immunogold analysis of trypanosomes expressing the FLAM8::YFP fusion protein stripped with detergent and stained with an anti-GFP antiserum. The signal is concentrated at the tip of microtubule doublets. A–C shows low magnification (bar is 1 μm) and squares indicate areas that were magnified in A_′–_C_′ (bar is 100 nm). D–G,_ IFA of methanol-fixed trypanosomes expressing the FLAM8::YFP fusion protein stained with an anti-GFP antiserum (green) and with the axonemal marker MAb25 (red) at various stages of flagellum elongation. The FLAM8 signal (D–G) was quantified and progressively accumulates at the distal tip of the new flagellum (yellow arrow) but its intensity is always lower compared with that at the distal tip of the old flagellum. Scale bar is 5 μm. H, I, The length of the new flagellum and the FLAM8::YFP signal intensity (H) or the difference between maximum and minimum intensity (I) were measured in 45 cells at different stages of the cell cycle and normalized by the equivalent parameter in the old flagellum of the same cell. The red and blue bars represent the flagellum length and the FLAM8 signal respectively.
Fig. 7.
Two different recovery profiles of FLAM8 signal after photobleaching. FRAP analysis of trypanosomes expressing the FLAM8::YFP fusion protein (see
supplemental Movie S1 and S2
). The distal tip signal was bleached with a brief laser pulse and recovery was monitored upon acquisition of an image every 30 s. Two profiles were observed, with six cells showing no detectable recovery (A) and six cells showing significant recovery (B). The bottom panels show magnification of the distal tip of the flagellum at the indicated time point.
Fig. 8.
Flagellar proteins show different turnover following RNAi silencing. Trypanosomes were transformed to express double-stranded RNA targeting AK1–3 (A–C), FLAM1 (D–F) or FLAM6 (G–I) leading to RNAi silencing. Cells were grown in the absence (non-induced, _A–A_′, _D–D_′, _G–G_′), or in the presence of tetracycline for 8 (_B–B_′), 24 (_C–C_′, _E–E_′, _F–F_′), or 48h (H–H′, _I–I_′) and analyzed by IFA following staining with the anti-arginine kinase antibody (white, A–C) in AK1–3RNAi cells; or with an anti-GFP in FLAM1RNAi cells expressing FLAM1::YFP (white, D–F) and in FLAM6RNAi cells expressing FLAM6::YFP (white, G–I). In these two cell lines, MAb25 was used to mark the axoneme (red) in double IFA with the anti-GFP (green, _D_′–_I_′). Only cells with two flagella are presented, showing equivalent bright staining between old and new flagella in non-induced samples. Whereas the arginine kinase signal goes down rapidly in both old and new flagella, the FLAM1::YFP and the FLAM6::YFP proteins disappear in the new flagellum but are still detected in the old flagellum (_F–F_′, _I–I_′). In intermediate situations, the signal is present at the proximal part of the flagellum but quickly becomes undetectable toward the end of the flagellum (yellow arrows on panels _E–E_′, _H–H_′), indicating polar assembly at the distal tip for both FLAM1 and FLAM6. Scale bar is 5 μm.
Similar articles
- Flagellar adhesion in Trypanosoma brucei relies on interactions between different skeletal structures in the flagellum and cell body.
Rotureau B, Blisnick T, Subota I, Julkowska D, Cayet N, Perrot S, Bastin P. Rotureau B, et al. J Cell Sci. 2014 Jan 1;127(Pt 1):204-15. doi: 10.1242/jcs.136424. Epub 2013 Oct 25. J Cell Sci. 2014. PMID: 24163437 - Flagellar incorporation of proteins follows at least two different routes in trypanosomes.
Vincensini L, Blisnick T, Bertiaux E, Hutchinson S, Georgikou C, Ooi CP, Bastin P. Vincensini L, et al. Biol Cell. 2018 Feb;110(2):33-47. doi: 10.1111/boc.201700052. Epub 2017 Dec 11. Biol Cell. 2018. PMID: 29148062 - Intraflagellar transport is required for the maintenance of the trypanosome flagellum composition but not its length.
Fort C, Bonnefoy S, Kohl L, Bastin P. Fort C, et al. J Cell Sci. 2016 Aug 1;129(15):3026-41. doi: 10.1242/jcs.188227. Epub 2016 Jun 24. J Cell Sci. 2016. PMID: 27343245 - The trypanosome flagellar pocket.
Field MC, Carrington M. Field MC, et al. Nat Rev Microbiol. 2009 Nov;7(11):775-86. doi: 10.1038/nrmicro2221. Epub 2009 Oct 6. Nat Rev Microbiol. 2009. PMID: 19806154 Review. - Cell-to-flagellum attachment and surface architecture in kinetoplastids.
de Liz LV, Stoco PH, Sunter JD. de Liz LV, et al. Trends Parasitol. 2023 May;39(5):332-344. doi: 10.1016/j.pt.2023.02.009. Epub 2023 Mar 16. Trends Parasitol. 2023. PMID: 36933967 Review.
Cited by
- Differential Subcellular Localization of Leishmania Alba-Domain Proteins throughout the Parasite Development.
Dupé A, Dumas C, Papadopoulou B. Dupé A, et al. PLoS One. 2015 Sep 3;10(9):e0137243. doi: 10.1371/journal.pone.0137243. eCollection 2015. PLoS One. 2015. PMID: 26334886 Free PMC article. - Light chain 2 is a Tctex-type related axonemal dynein light chain that regulates directional ciliary motility in Trypanosoma brucei.
Godar S, Oristian J, Hinsch V, Wentworth K, Lopez E, Amlashi P, Enverso G, Markley S, Alper JD. Godar S, et al. PLoS Pathog. 2022 Sep 26;18(9):e1009984. doi: 10.1371/journal.ppat.1009984. eCollection 2022 Sep. PLoS Pathog. 2022. PMID: 36155669 Free PMC article. - A cytoskeletal protein complex is essential for division of intracellular amastigotes of Leishmania mexicana.
Kelly FD, Tran KD, Hatfield J, Schmidt K, Sanchez MA, Landfear SM. Kelly FD, et al. J Biol Chem. 2020 Sep 11;295(37):13106-13122. doi: 10.1074/jbc.RA120.014065. Epub 2020 Jul 22. J Biol Chem. 2020. PMID: 32719012 Free PMC article. - Q586B2 is a crucial virulence factor during the early stages of Trypanosoma brucei infection that is conserved amongst trypanosomatids.
Stijlemans B, De Baetselier P, Van Molle I, Lecordier L, Hendrickx E, Romão E, Vincke C, Baetens W, Schoonooghe S, Hassanzadeh-Ghassabeh G, Korf H, Wallays M, Pinto Torres JE, Perez-Morga D, Brys L, Campetella O, Leguizamón MS, Claes M, Hendrickx S, Mabille D, Caljon G, Remaut H, Roelants K, Magez S, Van Ginderachter JA, De Trez C. Stijlemans B, et al. Nat Commun. 2024 Feb 27;15(1):1779. doi: 10.1038/s41467-024-46067-4. Nat Commun. 2024. PMID: 38413606 Free PMC article. - Characterization of a highly diverged mitochondrial ATP synthase Fo subunit in Trypanosoma brucei.
Dewar CE, Oeljeklaus S, Wenger C, Warscheid B, Schneider A. Dewar CE, et al. J Biol Chem. 2022 Apr;298(4):101829. doi: 10.1016/j.jbc.2022.101829. Epub 2022 Mar 12. J Biol Chem. 2022. PMID: 35293314 Free PMC article.
References
- Kohl L., Bastin P. (2005) The flagellum of trypanosomes. Int. Rev. Cytol. 244, 227–285 - PubMed
- Avidor-Reiss T., Maer A. M., Koundakjian E., Polyanovsky A., Keil T., Subramaniam S., Zuker C. S. (2004) Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell 117, 527–539 - PubMed
- Li J. B., Gerdes J. M., Haycraft C. J., Fan Y., Teslovich T. M., May-Simera H., Li H., Blacque O. E., Li L., Leitch C. C., Lewis R. A., Green J. S., Parfrey P. S., Leroux M. R., Davidson W. S., Beales P. L., Guay-Woodford L. M., Yoder B. K., Stormo G. D., Katsanis N., Dutcher S. K. (2004) Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell 117, 541–552 - PubMed
- Broadhead R., Dawe H. R., Farr H., Griffiths S., Hart S. R., Portman N., Shaw M. K., Ginger M. L., Gaskell S. J., McKean P. G., Gull K. (2006) Flagellar motility is required for the viability of the bloodstream trypanosome. Nature 440, 224–227 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases