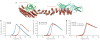Pharmacological chaperones stabilize retromer to limit APP processing - PubMed (original) (raw)
Pharmacological chaperones stabilize retromer to limit APP processing
Vincent J Mecozzi et al. Nat Chem Biol. 2014 Jun.
Abstract
Retromer is a multiprotein complex that trafficks cargo out of endosomes. The neuronal retromer traffics the amyloid-precursor protein (APP) away from endosomes, a site where APP is cleaved into pathogenic fragments in Alzheimer's disease. Here we determined whether pharmacological chaperones can enhance retromer stability and function. First, we relied on the crystal structures of retromer proteins to help identify the 'weak link' of the complex and to complete an in silico screen of small molecules predicted to enhance retromer stability. Among the hits, an in vitro assay identified one molecule that stabilized retromer against thermal denaturation. Second, we turned to cultured hippocampal neurons, showing that this small molecule increases the levels of retromer proteins, shifts APP away from the endosome, and decreases the pathogenic processing of APP. These findings show that pharmacological chaperones can enhance the function of a multiprotein complex and may have potential therapeutic implications for neurodegenerative diseases.
Conflict of interest statement
Competing financial interests
The authors declare no competing financial interests.
Figures
Figure 1. Characterization of the weak link in retromer complex stability
(a) Model of the core retromer complex based on electron microscopy data combined with crystal structures of the component proteins (reprinted with permission from ref. . The interaction of Vps35 (red and orange) with Vps29 (green) is known from a structure of their complex. The structure of the orange portion of Vps35 is hypothesized from sequence similarity to other helical solenoid-like proteins. The interaction between this portion of Vps35 and Vps26 (cyan) is based on modeling alone. (b) Thermal unfolding curve for purified, recombinant Vps35 C-terminal domain (blue curve), showing a denaturation temperature of 38.5 °C. Thermal unfolding curve for purified, recombinant Vps26 showing a denaturation temperature of 56.4 °C (red curve). (c) Thermal unfolding curve for the reconstituted complex of Vps35 and Vps26 (blue curve). The complex denatures at 53.4 °C. Thermal unfolding curve for the reconstituted complex of Vps35 and Vps29 (red curve). The melting temperature is not increased as much as when Vps26 binds Vps35. (d) Thermal unfolding curve for Vps29 in the absence and presence of zinc and manganese.
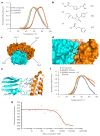
Figure 2. Identification of a retromer stabilizing pharmacological chaperone
(a) Thermal unfolding of the core retromer complex shown in the absence of any compound (blue), retromer in the presence of compound R53 identified from the virtual library (orange) and in the presence of the compound R55 (red) identified from the virtual screen. (b) Chemical structures of the compounds tested for pharmacological activity in vitro as well as in neuronal cell culture. (c) All of the potential binding sites identified computationally by searching for pockets with favorable steric and electrostatic properties were screened with our virtual library. The identified binding sites each gave Glide scores showing favorable binding to multiple compounds from our virtual library. (d) Docked structure of R55 with the Vps35–Vps29 heterodimer. The compound is predicted to bind at the interface between the two proteins, designated site 2 in Figure 2c. R55 is predicted to make interactions with side chains from both Vps35 (orange) and Vps29 (cyan). (e) Structure of site 2 of the Vps29–Vps35 interface shown with the Vps35 (orange) point mutation Q538W. The proposed mutation fills much of the proposed binding site for R55, which is also shown docked to the site. (f) Thermal unfolding curve of the WT retromer complex (blue), the Q538W mutant retromer (red), and the Q538W retromer in the presence of 20 mM R55 (orange). (g) Microscale thermophoresis scan of WT retromer titrated with R55 shows a _K_d of ~5 μM.
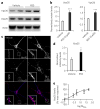
Figure 3. The pharmacological chaperone increases retromer levels in neurons
(a) Left panel, representative western blots of the effect of R55 (5 μM for 48 h) and vehicle on levels of Vps26 and Vps35 in 2-week-old primary cortical neuronal cultures derived from WT mice. (b) Quantitative analysis of the blots. Levels of Vps26 and Vps35 were normalized to total levels of actin (n = 9 per group, P < 0.001). Comparable results were obtained when tubulin was used a control (data not shown). (c) Primary hippocampal neurons were analyzed by confocal microscopy after labeling for MAP2 (blue) and Vps35 (red). Arrows indicate structures showing Vps35 labeling. Scale bars, 10 μm. (d) Bar graph showing a quantitative analysis of Vps35 intensity staining. Levels of Vps35 were normalized to total cell area (n = 14 cells per group). (e) Dose-response curve of the effect of R55 on Vps35 levels performed by western blot analysis (concentration of 0.5 μM, 2.5 μM, 5 μM, 20 μM and 50 μM were used, n = 8 per time point). Results here and in the following figures are shown as mean values ± s.e.m., where *P < 0.05, **P < 0.01 and ***P < 0.005, determined using Student’s _t_-test.
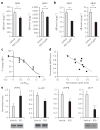
Figure 4. The pharmacological chaperone decreases Aβ peptide accumulation and reduces the pathogenic pathway of APP
(a) Effect of R55 (5 μM for 48 h) and vehicle on levels of endogenous mouse Aβ42 and Aβ40 in culture medium from WT primary cortical neurons (n = 12). Amount of Aβ is normalized to total protein content in neurons. (b) Effect of R55 (5 μM for 48 h) and vehicle on levels of human Aβ42 and Aβ40 in culture medium derived from J20 primary cortical neurons (n = 12). (c) Dose-response curve of the effect of R55 on endogenous Aβ production in hippocampal neurons (concentrations of 0.5 μM, 2.5 μM, 5 μM, 20 μM and 50 μM concentrations were used). Mouse and human Aβ samples were analyzed by ELISA. (d) Correlation analysis between R55 dose-dependent levels of neuronal Vps35 and Aβ42 production. Each point in the graph represents an individual measurement from the R55 dose-response curves for retromer and Aβ. (e) Quantification of APP metabolites in medium (sAPPα and sAPPβ) and in cell lysates (FL-APP and βCTF) in primary neurons exposed to R55 (5 μM) for 48 h. FL-APP, sAPPα and βCTF were measured by western blotting, and sAPPβ was measured by ELISA (n = 10 per fragment; Online Methods). *P < 0.05, **P < 0.01 and ***P < 0.005.
Figure 5. The pharmacological chaperone shifts the endosomal localization of APP and SorL1
(a) Top, representative confocal microscopy images of WT primary hippocampal neurons immunolabeled for MAP2, APP and EEA1 after a 48-h exposure to vehicle and R55. Insets show a magnification of representative dendritic areas used for the colocalization analysis of APP (red) and EEA1 (green). Arrows indicate structures showing colocalization between APP and EEA1. Bottom, confocal microscopy images of primary hippocampal neurons stained for MAP2, SorL1 and EEA1. Arrows indicate structures showing colocalization between SorL1 and EEA1. (b) Left, quantitative analysis of the colocalization of APP and EEA1 in dendrites (n = 19 cells per group). Right, quantification of colocalization between SorL1 and EEA1 (n = 14 cells per group). *P < 0.05, **P < 0.01.
Figure 6. The pharmacological chaperone specifically modulates Aβ production through the retromer pathway
(a) Representative western blots showing a knock-down of Vps26 (vehicle-Cre) in _Vps26_flox/flox neurons by the use of a lentivirus expressing CRE (three cultures shown for each condition, one per lane), n = 5 per group. (b) Quantitative analysis of the blots for Vps26 and Vps35 normalized to the vehicle-Δ condition. (c) Aβ42 levels in conditioned medium from neurons exposed to either vehicle or R55, and treated with either an active Cre virus (CRE) or the catalytically dead Cre virus (Δ). Aβ samples were analyzed by ELISA (Invitrogen) as suggested by the manufacturer. *P < 0.05.
Similar articles
- The location and trafficking routes of the neuronal retromer and its role in amyloid precursor protein transport.
Bhalla A, Vetanovetz CP, Morel E, Chamoun Z, Di Paolo G, Small SA. Bhalla A, et al. Neurobiol Dis. 2012 Jul;47(1):126-34. doi: 10.1016/j.nbd.2012.03.030. Epub 2012 Apr 6. Neurobiol Dis. 2012. PMID: 22516235 Free PMC article. - Retromer regulates postendocytic sorting of β-secretase in polarized Madin-Darby canine kidney cells.
Cuartero Y, Mellado M, Capell A, Alvarez-Dolado M, Verges M. Cuartero Y, et al. Traffic. 2012 Oct;13(10):1393-410. doi: 10.1111/j.1600-0854.2012.01392.x. Epub 2012 Jul 25. Traffic. 2012. PMID: 22758778 - Retromers in Alzheimer's disease.
Siegenthaler BM, Rajendran L. Siegenthaler BM, et al. Neurodegener Dis. 2012;10(1-4):116-21. doi: 10.1159/000335910. Epub 2012 Mar 3. Neurodegener Dis. 2012. PMID: 22398391 Review. - The use of pharmacological retromer chaperones in Alzheimer's disease and other endosomal-related disorders.
Berman DE, Ringe D, Petsko GA, Small SA. Berman DE, et al. Neurotherapeutics. 2015 Jan;12(1):12-8. doi: 10.1007/s13311-014-0321-y. Neurotherapeutics. 2015. PMID: 25472693 Free PMC article. Review. - Cross-linking of cell surface amyloid precursor protein leads to increased β-amyloid peptide production in hippocampal neurons: implications for Alzheimer's disease.
Lefort R, Pozueta J, Shelanski M. Lefort R, et al. J Neurosci. 2012 Aug 1;32(31):10674-85. doi: 10.1523/JNEUROSCI.6473-11.2012. J Neurosci. 2012. PMID: 22855816 Free PMC article.
Cited by
- PPAR-α agonist regulates amyloid-β generation via inhibiting BACE-1 activity in human neuroblastoma SH-SY5Y cells transfected with APPswe gene.
Zhang H, Gao Y, Qiao PF, Zhao FL, Yan Y. Zhang H, et al. Mol Cell Biochem. 2015 Oct;408(1-2):37-46. doi: 10.1007/s11010-015-2480-5. Epub 2015 Jun 20. Mol Cell Biochem. 2015. PMID: 26092426 - Identification of novel transplantable GPCR recycling motif for drug discovery.
Nooh MM, Mancarella S, Bahouth SW. Nooh MM, et al. Biochem Pharmacol. 2016 Nov 15;120:22-32. doi: 10.1016/j.bcp.2016.09.011. Epub 2016 Sep 16. Biochem Pharmacol. 2016. PMID: 27645110 Free PMC article. - Amyloid-β-independent regulators of tau pathology in Alzheimer disease.
van der Kant R, Goldstein LSB, Ossenkoppele R. van der Kant R, et al. Nat Rev Neurosci. 2020 Jan;21(1):21-35. doi: 10.1038/s41583-019-0240-3. Epub 2019 Nov 28. Nat Rev Neurosci. 2020. PMID: 31780819 Review. - VPS35 regulates parkin substrate AIMP2 toxicity by facilitating lysosomal clearance of AIMP2.
Yun SP, Kim H, Ham S, Kwon SH, Lee GH, Shin JH, Lee SH, Ko HS, Lee Y. Yun SP, et al. Cell Death Dis. 2017 Apr 6;8(4):e2741. doi: 10.1038/cddis.2017.157. Cell Death Dis. 2017. PMID: 28383562 Free PMC article. - Progress toward an integrated understanding of Parkinson's disease.
Rousseaux MWC, Shulman JM, Jankovic J. Rousseaux MWC, et al. F1000Res. 2017 Jul 12;6:1121. doi: 10.12688/f1000research.11820.1. eCollection 2017. F1000Res. 2017. PMID: 28751973 Free PMC article. Review.
References
- Seaman MN. Recycle your receptors with retromer. Trends Cell Biol. 2005;15:68–75. - PubMed
- Small SA, et al. Model-guided microarray implicates the retromer complex in Alzheimer’s disease. Ann Neurol. 2005;58:909–919. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases