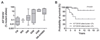The KIT D816V allele burden predicts survival in patients with mastocytosis and correlates with the WHO type of the disease - PubMed (original) (raw)
The KIT D816V allele burden predicts survival in patients with mastocytosis and correlates with the WHO type of the disease
G Hoermann et al. Allergy. 2014 Jun.
Abstract
KIT D816V is present in a majority of patients with systemic mastocytosis (SM). We determined the KIT D816V allele burden by quantitative real-time PCR in bone marrow and peripheral blood of 105 patients with mastocytosis. KIT D816V was detected in 92/105 patients (88%). Significant differences in the median allele burden were observed between disease subgroups: cutaneous mastocytosis (0.042%), indolent SM (0.285%), smoldering SM (5.991%), aggressive SM (9.346%), and SM with associated hematologic non-mast cell lineage disease (3.761%) (P < 0.001). The KIT D816V burden also correlated with serum tryptase (R = 0.5, P < 0.005) but not with mast cell infiltration in bone marrow or mediator symptoms. Moreover, the allele burden was of prognostic significance regarding survival (P < 0.01). Patients responding to cytoreductive therapy showed a significant decrease in KIT D816V (P < 0.05). To conclude, the KIT D816V burden correlates with the variant of mastocytosis, predicts survival, and is a valuable follow-up parameter in SM.
Keywords: KIT D816V; allele burden; mastocytosis; survival; treatment response.
© 2014 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of Interest
P.V. is a consultant in a global Novartis trial investigating the effects of PKC412 in patients with advanced systemic mastocytosis. In addition, P.V. received honoraria and a research grant from Novartis. The authors declare no other competing financial interests.
Figures
Fig. 1. KIT D816V allele burden in different WHO-subtype of mastocytosis and its impact on survival.
(A) Highly significant differences in the KIT D816V allele burden were found between patients with cutaneous mastocytosis (CM), mastocytosis in the skin (MIS), indolent systemic mastocytosis (ISM), smoldering systemic mastocytosis (SSM), aggressive systemic mastocytosis (ASM), mast cell leukemia (MCL), and systemic mastocytosis with an associated hematologic non-mast cell lineage disease (SM-AHNMD) (P<0.001, Kruskal Wallis test). (B) Kaplan-Meier plot for overall survival of mastocytosis patients with a KIT D816V mutation burden <2% and patients with a KIT D816V burden of ≥ 2% at diagnosis. The difference in the probability of survival was significant (_P_=0.001).
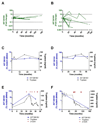
Fig. 2. KIT D816V allele burden during the follow-up.
Change in KIT D816V mutation positive allele fraction during the follow-up in patients without (A; _N_=19) and with cytoreductive treatment (B; _N_=11). The allele burden was normalized to the mutant burden at diagnosis. The value of 1 corresponds to no change in allele fraction over time and the values of 0.1 and 10 (dotted lines) correspond to 10 fold decreases and increases. (C-F) Follow-up of KIT D816V allele burden (blue) and serum tryptase (black) in individual mastocytosis patients with an uneventful course (C, ISM; D, SSM), a patient initially diagnosed as ISM with disease progression to ASM-CMML and response to cytoreductive therapy with cladribine (2-CDA) (E), and a patient with SSM receiving cytoreductive therapy due to recurrent episodes of life threatening vascular instability requiring epinephrine support (F).
Similar articles
- Serum tryptase correlates with the KIT D816V mutation burden in adults with indolent systemic mastocytosis.
Kristensen T, Broesby-Olsen S, Vestergaard H, Bindslev-Jensen C, Møller MB; Mastocytosis Centre Odense University Hospital. Kristensen T, et al. Eur J Haematol. 2013 Aug;91(2):106-11. doi: 10.1111/ejh.12128. Epub 2013 Jun 28. Eur J Haematol. 2013. PMID: 23621866 - The KIT D816V expressed allele burden for diagnosis and disease monitoring of systemic mastocytosis.
Erben P, Schwaab J, Metzgeroth G, Horny HP, Jawhar M, Sotlar K, Fabarius A, Teichmann M, Schneider S, Ernst T, Müller MC, Giehl M, Marx A, Hartmann K, Hochhaus A, Hofmann WK, Cross NC, Reiter A. Erben P, et al. Ann Hematol. 2014 Jan;93(1):81-8. doi: 10.1007/s00277-013-1964-1. Epub 2013 Nov 27. Ann Hematol. 2014. PMID: 24281161 Clinical Trial. - Digital PCR: A Sensitive and Precise Method for KIT D816V Quantification in Mastocytosis.
Greiner G, Gurbisz M, Ratzinger F, Witzeneder N, Simonitsch-Klupp I, Mitterbauer-Hohendanner G, Mayerhofer M, Müllauer L, Sperr WR, Valent P, Hoermann G. Greiner G, et al. Clin Chem. 2018 Mar;64(3):547-555. doi: 10.1373/clinchem.2017.277897. Epub 2017 Dec 13. Clin Chem. 2018. PMID: 29237714 Free PMC article. - KIT Mutations and Other Genetic Defects in Mastocytosis: Implications for Disease Pathology and Targeted Therapies.
Chantran Y, Valent P, Arock M. Chantran Y, et al. Immunol Allergy Clin North Am. 2023 Nov;43(4):651-664. doi: 10.1016/j.iac.2023.04.008. Epub 2023 Jun 11. Immunol Allergy Clin North Am. 2023. PMID: 37758404 Review. - New Insights into the Pathogenesis of Mastocytosis: Emerging Concepts in Diagnosis and Therapy.
Valent P, Akin C, Sperr WR, Horny HP, Arock M, Metcalfe DD, Galli SJ. Valent P, et al. Annu Rev Pathol. 2023 Jan 24;18:361-386. doi: 10.1146/annurev-pathmechdis-031521-042618. Epub 2022 Oct 21. Annu Rev Pathol. 2023. PMID: 36270293 Review.
Cited by
- Systemic Mastocytosis: Molecular Landscape and Implications for Treatment.
Monaldi C, De Santis S, Mancini M, Bruno S, Cavo M, Soverini S. Monaldi C, et al. Mediterr J Hematol Infect Dis. 2021 Jul 1;13(1):e2021046. doi: 10.4084/MJHID.2021.046. eCollection 2021. Mediterr J Hematol Infect Dis. 2021. PMID: 34276915 Free PMC article. Review. - Mastocytosis.
Akin C, Arock M, Carter MC, George TI, Valent P. Akin C, et al. Nat Rev Dis Primers. 2025 Apr 24;11(1):30. doi: 10.1038/s41572-025-00611-8. Nat Rev Dis Primers. 2025. PMID: 40274818 Review. - Management of Advanced Systemic Mastocytosis: Clinical Challenges.
Tremblay D, Wagner NE, Mascarenhas J. Tremblay D, et al. J Blood Med. 2024 Sep 11;15:421-433. doi: 10.2147/JBM.S366367. eCollection 2024. J Blood Med. 2024. PMID: 39279879 Free PMC article. Review. - Recent Advances in the Molecular Biology of Systemic Mastocytosis: Implications for Diagnosis, Prognosis, and Therapy.
Martelli M, Monaldi C, De Santis S, Bruno S, Mancini M, Cavo M, Soverini S. Martelli M, et al. Int J Mol Sci. 2020 Jun 2;21(11):3987. doi: 10.3390/ijms21113987. Int J Mol Sci. 2020. PMID: 32498255 Free PMC article. Review. - Unraveling the Rare Entity of KIT D816V-Negative Systemic Mastocytosis.
Alyamany R, Albachir CA, Alsaleh S, Hamad A, Abdulwali SK, Alotaibi AS, Ahmed SO, Alfayez M. Alyamany R, et al. J Hematol. 2024 Jun;13(3):128-136. doi: 10.14740/jh1279. Epub 2024 Jun 28. J Hematol. 2024. PMID: 38993735 Free PMC article.
References
- Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y, et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci U S A. 1995;92(23):10560–10564. - PMC - PubMed
- Valent P, Akin C, Escribano L, Fodinger M, Hartmann K, Brockow K, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37(6):435–453. - PubMed
- Gotlib J, Pardanani A, Akin C, Reiter A, George T, Hermine O, et al. International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) & European Competence Network on Mastocytosis (ECNM) consensus response criteria in advanced systemic mastocytosis. Blood. 2013;121(13):2393–2401. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases