Breaching the nuclear envelope in development and disease - PubMed (original) (raw)
Review
Breaching the nuclear envelope in development and disease
Emily Hatch et al. J Cell Biol. 2014.
Abstract
In eukaryotic cells the nuclear genome is enclosed by the nuclear envelope (NE). In metazoans, the NE breaks down in mitosis and it has been assumed that the physical barrier separating nucleoplasm and cytoplasm remains intact during the rest of the cell cycle and cell differentiation. However, recent studies suggest that nonmitotic NE remodeling plays a critical role in development, virus infection, laminopathies, and cancer. Although the mechanisms underlying these NE restructuring events are currently being defined, one common theme is activation of protein kinase C family members in the interphase nucleus to disrupt the nuclear lamina, demonstrating the importance of the lamina in maintaining nuclear integrity.
Figures
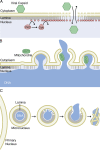
Figure 1.
Nuclear envelope budding of export complexes. (A) Herpes virus capsids bind to viral proteins at the INM that also recruit PKC. Viral capsids then bud through the envelope at sites of lamina disorganization (gray) and are released into the cytoplasm. (B) mRNP export in differentiating muscle cells also requires disorganization of the nuclear lamina by PKC. mRNPs interact with the INM at sites of lamina disorganization and bud into the perinuclear space with the help of torsinA. The perinuclear vesicle fuses with the ONM and the mRNP is released into the cytoplasm.
Figure 2.
Nuclear envelope rupturing and collapse. (A) Association of parvovirus capsids with the ONM causes breakdown of first the outer and then the inner nuclear membranes. Activation of PKC and Cdk kinases in the nucleus during this time forms large gaps in the lamina, allowing the capsids to enter the nucleoplasm and causing a loss of nuclear integrity. (B) When lamina organization is disrupted by changes in lamina proteins, patches of weak membrane form and chromatin can herniate. This membrane can undergo multiple rounds of NE rupturing and repair, causing mislocalization and entrapment of cytosolic and nuclear components. (C) Micronuclei have a high probability of NE rupturing but fail to undergo NE repair, causing a persistent loss of nuclear integrity. After rupturing, the chromatin forms aberrant associations with ER tubules in a process called NE collapse.
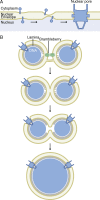
Figure 3.
Nuclear envelope fusion. (A) An early step in interphase nuclear pore assembly is the formation of a channel through the NE by fusion of the inner and outer nuclear membranes. Membrane-associated proteins are thought to bend the membranes toward each other and stabilize the channel before nuclear pore assembly. (B) During fertilization and early embryonic divisions, multiple nuclei can form and must fuse into a mononucleus. Although the nuclear membranes are part of the same endomembrane system, specific proteins, including brambleberry, are required to initiate fusion. Full fusion requires mixing of the outer and inner nuclear membranes and, likely, disorganization of the lamina to expand the fusion pore.
Similar articles
- Function of nuclear membrane proteins in shaping the nuclear envelope integrity during closed mitosis.
Yang HJ, Iwamoto M, Hiraoka Y, Haraguchi T. Yang HJ, et al. J Biochem. 2017 Jun 1;161(6):471-477. doi: 10.1093/jb/mvx020. J Biochem. 2017. PMID: 28398483 Review. - Breaking down the wall: the nuclear envelope during mitosis.
Smoyer CJ, Jaspersen SL. Smoyer CJ, et al. Curr Opin Cell Biol. 2014 Feb;26:1-9. doi: 10.1016/j.ceb.2013.08.002. Epub 2013 Sep 4. Curr Opin Cell Biol. 2014. PMID: 24529240 Review. - Nuclear pore biogenesis into an intact nuclear envelope.
Doucet CM, Hetzer MW. Doucet CM, et al. Chromosoma. 2010 Oct;119(5):469-77. doi: 10.1007/s00412-010-0289-2. Epub 2010 Aug 19. Chromosoma. 2010. PMID: 20721671 Review. - Crosstalk between mitotic reassembly and repair of the nuclear envelope.
Kono Y, Shimi T. Kono Y, et al. Nucleus. 2024 Dec;15(1):2352203. doi: 10.1080/19491034.2024.2352203. Epub 2024 May 23. Nucleus. 2024. PMID: 38780365 Free PMC article. Review. - Loss of Nuclear Envelope Integrity in Aging and Disease.
Robijns J, Houthaeve G, Braeckmans K, De Vos WH. Robijns J, et al. Int Rev Cell Mol Biol. 2018;336:205-222. doi: 10.1016/bs.ircmb.2017.07.013. Epub 2017 Sep 18. Int Rev Cell Mol Biol. 2018. PMID: 29413891 Review.
Cited by
- Recent progress in the mechanistic understanding of NET formation in neutrophils.
Liu ML, Lyu X, Werth VP. Liu ML, et al. FEBS J. 2022 Jul;289(14):3954-3966. doi: 10.1111/febs.16036. Epub 2021 Jun 11. FEBS J. 2022. PMID: 34042290 Free PMC article. Review. - AIM2 inflammasome is activated by pharmacological disruption of nuclear envelope integrity.
Di Micco A, Frera G, Lugrin J, Jamilloux Y, Hsu ET, Tardivel A, De Gassart A, Zaffalon L, Bujisic B, Siegert S, Quadroni M, Broz P, Henry T, Hrycyna CA, Martinon F. Di Micco A, et al. Proc Natl Acad Sci U S A. 2016 Aug 9;113(32):E4671-80. doi: 10.1073/pnas.1602419113. Epub 2016 Jul 26. Proc Natl Acad Sci U S A. 2016. PMID: 27462105 Free PMC article. - ESCRT machinery: Damage control at the nuclear membrane.
Ventimiglia LN, Martin-Serrano J. Ventimiglia LN, et al. Cell Res. 2016 Jun;26(6):641-2. doi: 10.1038/cr.2016.52. Epub 2016 May 6. Cell Res. 2016. PMID: 27151367 Free PMC article. - The LEM-ESCRT toolkit: Repair and maintenance of the nucleus.
Borah S, Dhanasekaran K, Kumar S. Borah S, et al. Front Cell Dev Biol. 2022 Sep 12;10:989217. doi: 10.3389/fcell.2022.989217. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36172278 Free PMC article. Review. - Bursting the Bubble - Nuclear Envelope Rupture as a Path to Genomic Instability?
Shah P, Wolf K, Lammerding J. Shah P, et al. Trends Cell Biol. 2017 Aug;27(8):546-555. doi: 10.1016/j.tcb.2017.02.008. Epub 2017 Mar 9. Trends Cell Biol. 2017. PMID: 28285738 Free PMC article. Review.
References
- Bakheet S.A., Attia S.M., Al-Rasheed N.M., Al-Harbi M.M., Ashour A.E., Korashy H.M., Abd-Allah A.R., Saquib Q., Al-Khedhairy A.A., Musarrat J. 2011. Salubrious effects of dexrazoxane against teniposide-induced DNA damage and programmed cell death in murine marrow cells. Mutagenesis. 26:533–543 10.1093/mutage/ger013 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources