Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices - PubMed (original) (raw)
Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices
Daniel J Chandler et al. Proc Natl Acad Sci U S A. 2014.
Abstract
The brainstem nucleus locus coeruleus (LC) is the primary source of norepinephrine (NE) to the mammalian neocortex. It is believed to operate as a homogeneous syncytium of transmitter-specific cells that regulate brain function and behavior via an extensive network of axonal projections and global transmitter-mediated modulatory influences on a diverse assembly of neural targets within the CNS. The data presented here challenge this longstanding notion and argue instead for segregated operation of the LC-NE system with respect to the functions of the circuits within its efferent domain. Anatomical, molecular, and electrophysiological approaches were used in conjunction with a rat model to show that LC cells innervating discrete cortical regions are biochemically and electrophysiologically distinct from one another so as to elicit greater release of norepinephrine in prefrontal versus motor cortex. These findings challenge the consensus view of LC as a relatively homogeneous modulator of forebrain activity and have important implications for understanding the impact of the system on the generation and maintenance of adaptive and maladaptive behaviors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
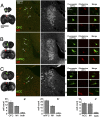
LC cells innervating M1 are distinct from those innervating OFC, mPFC, and ACC. (A_–_C) Representative photomicrographs of pairs of injection sites (Left) and LC through merged fluorescence filters to detect retrograde tracers (second from Left) and DBH immunofluorescence (third from Left) following injections into OFC and M1 (A), mPFC and M1 (B), and ACC and M1 (C). Arrowheads and Roman numerals indicate the locations of cells shown in high power images in the Right panels. Columns show individual neuron(s) through fluorescein (OFC, mPFC, or ACC), rhodamine (M1), and merged fluorescence filters, thus identifying single labeled cells with different terminal fields. (_A_′–_C_′) Histograms show mean cell counts per animal ± SEM projecting to each possible combination of terminal fields (n = 5 rats per experiment).
Fig. 2.
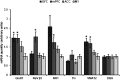
LC cells projecting to OFC and mPFC contain enriched mRNA transcripts coding for markers of excitability and release. Mean ± SEM of relative quantities of mRNAs that were shown to differ significantly from M1 projection cells according to terminal field by a Kruskal–Wallis H test (*P < 0.05). DBH is shown in the far right as a representation of protein whose mRNA expression was highly consistent between populations.
Fig. 3.
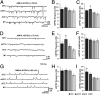
EPSC size and frequency vary according to LC neuronal terminal field. Representative traces of AMPA-mediated sEPSCs, mEPSCs, and NMDA-mediated sEPSCs are shown in A, D, and G. Mean frequencies and amplitudes of these events are shown in B, E, and H, and C, F, and I, respectively. Cells projecting to mPFC were characterized by significantly larger AMPA-mediated sEPSCs and more frequent mEPSCs than those projecting to M1, and cells projecting to OFC were characterized by significantly larger NMDA-mediated sEPSCs than M1 projection cells. Values were generated by using an automated search protocol in ClampFit software to identify all sEPSC events recorded from each cell and calculate a single mean sEPSC size for each cell. Any event smaller than twice the SD of the baseline of the recording was discarded. These data were then used to generate mean ± SEM amplitude values on a cell by cell basis. *Significantly different from M1 projection cells (P < 0.05).
Fig. 4.
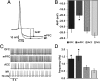
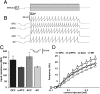
LC cells projecting to mPFC are more spontaneously active and have a smaller AHP than those projecting to M1. (A) Representative traces of individual action potentials from an mPFC and M1 projection cell showing the magnitude of afterhyperpolarization as measured from action potential threshold to its most hyperpolarized state (dashed lines and arrows). Mean ± SEM afterhyperpolarization amplitudes are shown in B. (C) Representative traces of spontaneous action potentials from all populations of LC projection cells (OFC, n = 18; mPFC, n = 19; ACC, n = 18; M1, n = 17). Mean ± SEM of spontaneous discharge values are shown in D. *Significantly different from M1 projection cells (P < 0.05).
Fig. 5.
LC cells projecting to mPFC are more excitable than those projecting to M1. (A) Patched cells were injected with a −0.1-nA current step to measure input resistance, followed by a series of progressively increasing current steps (−3.0 to +4.0 nA, 0.5-nA interval). Representative traces of discharge in response to a 1-s injection of 4.0 nA are shown in B. Action potentials were truncated 5 mV above firing threshold. (C) Input resistance was significantly greater in cells projecting to OFC and ACC than M1. (Inset) The responses to the current injection from representative traces shown in B superimposed on one another. Note that the larger deflections in voltage are from ACC and OFC projection cells whereas the smaller two are from M1 and mPFC projection cells (P < 0.05). A plot of the mean ± SEM of the number of action potentials fired in response to all levels of current injected into each of the populations of LC neurons is shown in D. *Significantly different from M1 projection cells (P < 0.05).
Fig. 6.
Cells projecting to PFC and M1 segregate from one another in principal-component space. Principal-component analysis was used to construct composite variables from several current-clamp parameters measured independently. Regression scores were calculated in IBM SPSS Statistics version 20. Scores for PC1 versus PC2 are plotted for all cells in all groups. Note that cells projecting to OFC and ACC differ from M1 projection cells primarily in PC2 (membrane properties) whereas cells projecting to mPFC differ mostly from cells projecting to M1 in PC1 (action potential properties). Individual weights of each of the current-clamp measures to each principal component are shown in
Table S6
. The quality of cluster separation was evaluated using the Davies–Bouldin index (45). Computations were performed in the R language (46) using the clusterSim package according to Walesiak and Dudek (47), with cluster dispersion assessed as the SD of distances from points to the cluster centroid, and separation between clusters assessed as the Euclidean distance. Davies–Bouldin index values for comparisons between M1 and OFC, mPFC, and ACC were 1.47, 1.13, and 1.40, respectively.
Similar articles
- Locus coeruleus phasic discharge is essential for stimulus-induced gamma oscillations in the prefrontal cortex.
Neves RM, van Keulen S, Yang M, Logothetis NK, Eschenko O. Neves RM, et al. J Neurophysiol. 2018 Mar 1;119(3):904-920. doi: 10.1152/jn.00552.2017. Epub 2017 Nov 1. J Neurophysiol. 2018. PMID: 29093170 - Probing the structure and function of locus coeruleus projections to CNS motor centers.
Waterhouse BD, Predale HK, Plummer NW, Jensen P, Chandler DJ. Waterhouse BD, et al. Front Neural Circuits. 2022 Sep 29;16:895481. doi: 10.3389/fncir.2022.895481. eCollection 2022. Front Neural Circuits. 2022. PMID: 36247730 Free PMC article. - Locus coeruleus: From global projection system to adaptive regulation of behavior.
Aston-Jones G, Waterhouse B. Aston-Jones G, et al. Brain Res. 2016 Aug 15;1645:75-8. doi: 10.1016/j.brainres.2016.03.001. Epub 2016 Mar 9. Brain Res. 2016. PMID: 26969408 Free PMC article. Review. - Evidence for a specialized role of the locus coeruleus noradrenergic system in cortical circuitries and behavioral operations.
Chandler DJ. Chandler DJ. Brain Res. 2016 Jun 15;1641(Pt B):197-206. doi: 10.1016/j.brainres.2015.11.022. Epub 2015 Nov 25. Brain Res. 2016. PMID: 26607255 Free PMC article. Review.
Cited by
- Recurrent Hippocampo-neocortical sleep-state divergence in humans.
Jang RS, Ciliberti D, Mankin EA, Poe GR. Jang RS, et al. Proc Natl Acad Sci U S A. 2022 Nov;119(44):e2123427119. doi: 10.1073/pnas.2123427119. Epub 2022 Oct 24. Proc Natl Acad Sci U S A. 2022. PMID: 36279474 Free PMC article. - In vivo cell type-specific CRISPR knockdown of dopamine beta hydroxylase reduces locus coeruleus evoked wakefulness.
Yamaguchi H, Hopf FW, Li SB, de Lecea L. Yamaguchi H, et al. Nat Commun. 2018 Dec 6;9(1):5211. doi: 10.1038/s41467-018-07566-3. Nat Commun. 2018. PMID: 30523254 Free PMC article. - The Aversive Lens: Stress effects on the prefrontal-cingulate cortical pathways that regulate emotion.
Arnsten AFT, Joyce MKP, Roberts AC. Arnsten AFT, et al. Neurosci Biobehav Rev. 2023 Feb;145:105000. doi: 10.1016/j.neubiorev.2022.105000. Epub 2022 Dec 15. Neurosci Biobehav Rev. 2023. PMID: 36529312 Free PMC article. Review. - The Contribution of the Locus Coeruleus-Noradrenaline System Degeneration during the Progression of Alzheimer's Disease.
Mercan D, Heneka MT. Mercan D, et al. Biology (Basel). 2022 Dec 14;11(12):1822. doi: 10.3390/biology11121822. Biology (Basel). 2022. PMID: 36552331 Free PMC article. Review. - The Neuromodulatory Role of the Noradrenergic and Cholinergic Systems and Their Interplay in Cognitive Functions: A Focused Review.
Slater C, Liu Y, Weiss E, Yu K, Wang Q. Slater C, et al. Brain Sci. 2022 Jul 7;12(7):890. doi: 10.3390/brainsci12070890. Brain Sci. 2022. PMID: 35884697 Free PMC article. Review.
References
- Amaral DG, Sinnamon HM. The locus coeruleus: Neurobiology of a central noradrenergic nucleus. Prog Neurobiol. 1977;9(3):147–196. - PubMed
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10(3):211–223. - PubMed
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42(1):33–84. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources