Single-molecule imaging of FtsK translocation reveals mechanistic features of protein-protein collisions on DNA - PubMed (original) (raw)
Single-molecule imaging of FtsK translocation reveals mechanistic features of protein-protein collisions on DNA
Ja Yil Lee et al. Mol Cell. 2014.
Abstract
In physiological settings, DNA translocases will encounter DNA-bound proteins, which must be dislodged or bypassed to allow continued translocation. FtsK is a bacterial translocase that promotes chromosome dimer resolution and decatenation by activating XerCD-dif recombination. To better understand how translocases act in crowded environments, we used single-molecule imaging to visualize FtsK in real time as it collided with other proteins. We show that FtsK can push, evict, and even bypass DNA-bound proteins. The primary factor dictating the outcome of collisions was the relative affinity of the proteins for their specific binding sites. Importantly, protein-protein interactions between FtsK and XerD help prevent removal of XerCD from DNA by promoting rapid reversal of FtsK. Finally, we demonstrate that RecBCD always overwhelms FtsK when these two motor proteins collide while traveling along the same DNA molecule, indicating that RecBCD is capable of exerting a much greater force than FtsK when translocating along DNA.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
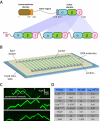
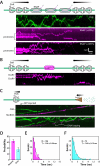
Figure 1. Experimental system for visualizing FtsK protein-protein collisions on DNA. (A)
Overview of the linked trimeric FtsKαβγ construct (Crozat et al., 2010). (B) Schematic illustration of the double-tethered DNA curtains used to assay FtsKαβγ translocation activity. (C) Examples of kymographs highlighting typical examples of QD-tagged FtsKαβγ (shown in green) translocating on unlabeled DNA substrates; the DNA is unlabeled because intercalating dyes such as YOYO1 inhibit the translocation of FtsK (Lee et al., 2012). (D) List of roadblock proteins indicating the experimentally determined Kd and koff values based on bulk biochemical DNA binding measurements.
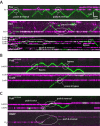
Figure 2. Collisions between single FtsK motors and stationary DNA binding proteins. (A)
Kymographs highlighting examples of FtsKαβγ (shown in green) pauses and reversals upon colliding with either Tus, LacI, or EcoRIE111Q (shown in magenta) bound to TerB, LacO, or EcoRI cognate sites, respectively. (B) Kymographs highlighting examples of protein bypass by FtsKαβγ during collisions with either the ATPase mutant FtsKD1121A bound to a 3xKOPS site, or EcoRIE111Q bound to its cognate site. (C) Kymographs highlighting examples of either FtsKD1121A or RNAP being pushed by FtsKαβγ.
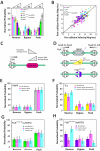
Figure 3. Collision outcome is dictated by obstacle protein binding affinity. (A)
FtsKαβγ collision outcomes summarized for each of the different (non-Xer) obstacle proteins. The data within each category (reverse, bypass, and push) are organized according to the binding affinity of each protein for its specific site, as indicated. (B) FtsKαβγs velocity before collisions versus velocity while pushing the obstacle protein; the back line represents reference slope of 1. (C) Model summarizing effect of protein DNA-binding affinity on the ability of FtsK to remove it from its binding site. (D) Schematic illustration of the different potential collision orientations for RNAP, Tus, and FtsKD1121A. Collision outcomes segregated based on protein orientation for (E) RNAP, (F) Tus, and (G) FtsKD1121A bound to either a single KOPS or (H) a triple KOPS site.
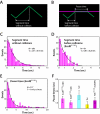
Figure 4. FtsK collisions with different proteins results in uniform pause times. (A)
Schematic illustrating defining how the segment times are determined from translocation trajectories on naked DNA, and (B) how segment times and pause times are defined in the presence of other DNA-binding proteins. (C) FtsKαβγ segment times on naked DNA. (D) Segment times before collisions with EcoRIE111Q; segment times were only obtained from DNA molecules with a single bound obstacle protein to avoid biasing the data towards shorter time intervals. (E) Pause time distribution graph for FtsKαβγ collisions with EcoRIE111Q. (F) FtsKαβγ pause times for collisions with different obstacle proteins; pause times could not be determined for collisions with RNAP because FtsKαβγ typically pushed RNAP rather than pausing.
Figure 5. Collisions with XerD provoke rapid reversal of FtsK. (A)
Kymographs of FtsKαβγ collisions with XerD (upper panel) and XerCD (lower panel), as indicated. (B) FtsKαβγ-induced displacement probability versus koff in the absence of FtsKαβγ for each of the different obstacle proteins. The dashed line indicates a linear fit to all the data for the non-Xer proteins. Square data points were collected using FtsKαβγ and circular data points were from experiments using FtsKΔγ. (C) Schematic illustrating potential collision orientations for reactions with XerD and XerCD; note that in reactions with XerD only we cannot experimentally distinguish between XerD monomers and XerD dimers. (D) Event outcomes for XerD collisions segregated for orientation. (E) Event outcomes for XerCD collisions segregated for orientation. (F) FtsKαβγ pause lifetimes during collisions with XerD and XerCD segregated for orientation and compared to the pause lifetimes of all other non-Xer roadblock proteins. (G) Examples of collisions between FtsKΔγ and XerD, and (H) collision outcomes segregated by protein orientation.
Figure 6. The combined action of multiple FtsK motors results in rapid removal of obstacle proteins from DNA. (A)
Kymograph showing an example of QD-tagged FtsKαβγ (upper panel) at higher protein concentrations and revealing an average of ~5-10 labeled FtsKαβγ motors per DNA. Kymographs showing the removal of promoter-bound RNAP (middle panel) and RNAP elongation complexes (lower panel) (shown in magenta) by FtsKαβγ (unlabeled). Kymographs showing that higher concentrations of FtsK also result in the removal of (B) EcoRIE111Q. (C) Kymograph showing collisions between FtsKαβγ and single RecBCD complexes. Abrupt increases in the apparent velocity of RecBCD correspond to FtsKαβγ-induced DNA-looping events, as highlighted. (D) Probability of FtsKαβγ reversal and eviction during collisions with RecBCD. (E) Lifetime of FtsKαβγ at RecBCD prior to reversing the direction of translocation. (F) Lifetime of FtsKαβγ at RecBCD prior to being evicted from the DNA.
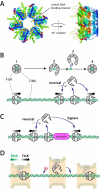
Figure 7. Model for FtsK reversal and protein bypass. (A)
Crystal structure of the FtsKαβ hexamer from P. aeruginosa highlighting the DNA binding channel (Massey et al., 2006). (B) Model for FtsKαβγ reversal, in which the hexameric ring opens, allowing the protein to microscopically dissociate from the DNA without fully equilibrating into free solution. Re-association with the DNA can occur in either of two possible orientations allowing the protein to spontaneously reverse direction. (C) The same mechanism involving a microscopically dissociated FtsKαβγ intermediate provides an explanation for how FtsK might bypass DNA-bound obstacles. (D) Physical constraints imposed by the division septum may be sufficient to prevent FtsK from reversing orientation during chromosome segregation.
Similar articles
- FtsK translocation on DNA stops at XerCD-dif.
Graham JE, Sivanathan V, Sherratt DJ, Arciszewska LK. Graham JE, et al. Nucleic Acids Res. 2010 Jan;38(1):72-81. doi: 10.1093/nar/gkp843. Epub 2009 Oct 23. Nucleic Acids Res. 2010. PMID: 19854947 Free PMC article. - Assembly, translocation, and activation of XerCD-dif recombination by FtsK translocase analyzed in real-time by FRET and two-color tethered fluorophore motion.
May PF, Zawadzki P, Sherratt DJ, Kapanidis AN, Arciszewska LK. May PF, et al. Proc Natl Acad Sci U S A. 2015 Sep 15;112(37):E5133-41. doi: 10.1073/pnas.1510814112. Epub 2015 Aug 31. Proc Natl Acad Sci U S A. 2015. PMID: 26324908 Free PMC article. - Activation of XerCD-dif recombination by the FtsK DNA translocase.
Grainge I, Lesterlin C, Sherratt DJ. Grainge I, et al. Nucleic Acids Res. 2011 Jul;39(12):5140-8. doi: 10.1093/nar/gkr078. Epub 2011 Mar 2. Nucleic Acids Res. 2011. PMID: 21371996 Free PMC article. - The Escherichia coli DNA translocase FtsK.
Sherratt DJ, Arciszewska LK, Crozat E, Graham JE, Grainge I. Sherratt DJ, et al. Biochem Soc Trans. 2010 Apr;38(2):395-8. doi: 10.1042/BST0380395. Biochem Soc Trans. 2010. PMID: 20298190 Review. - FtsK DNA translocase: the fast motor that knows where it's going.
Crozat E, Grainge I. Crozat E, et al. Chembiochem. 2010 Nov 2;11(16):2232-43. doi: 10.1002/cbic.201000347. Chembiochem. 2010. PMID: 20922738 Review.
Cited by
- Deciphering Complexity in Molecular Biophysics with Single-Molecule Resolution.
Deniz AA. Deniz AA. J Mol Biol. 2016 Jan 29;428(2 Pt A):301-307. doi: 10.1016/j.jmb.2015.12.011. Epub 2015 Dec 19. J Mol Biol. 2016. PMID: 26707199 Free PMC article. Review. - Xer Site Specific Recombination: Double and Single Recombinase Systems.
Castillo F, Benmohamed A, Szatmari G. Castillo F, et al. Front Microbiol. 2017 Mar 20;8:453. doi: 10.3389/fmicb.2017.00453. eCollection 2017. Front Microbiol. 2017. PMID: 28373867 Free PMC article. Review. - Structural basis of nucleosome assembly by the Abo1 AAA+ ATPase histone chaperone.
Cho C, Jang J, Kang Y, Watanabe H, Uchihashi T, Kim SJ, Kato K, Lee JY, Song JJ. Cho C, et al. Nat Commun. 2019 Dec 17;10(1):5764. doi: 10.1038/s41467-019-13743-9. Nat Commun. 2019. PMID: 31848341 Free PMC article. - How Xer-exploiting mobile elements overcome cellular control.
Midonet C, Barre FX. Midonet C, et al. Proc Natl Acad Sci U S A. 2016 Jul 26;113(30):8343-5. doi: 10.1073/pnas.1608539113. Epub 2016 Jul 15. Proc Natl Acad Sci U S A. 2016. PMID: 27422553 Free PMC article. No abstract available. - Two-step chromosome segregation in the stalked budding bacterium Hyphomonas neptunium.
Jung A, Raßbach A, Pulpetta RL, van Teeseling MCF, Heinrich K, Sobetzko P, Serrania J, Becker A, Thanbichler M. Jung A, et al. Nat Commun. 2019 Jul 23;10(1):3290. doi: 10.1038/s41467-019-11242-5. Nat Commun. 2019. PMID: 31337764 Free PMC article.
References
- Aussel L, Barre F, Aroyo M, Stasiak A, Stasiak A, Sherratt D. FtsK Is a DNA motor protein that activates chromosome dimer resolution by switching the catalytic state of the XerC and XerD recombinases. Cell. 2002;108:195–205. - PubMed
- Barre F. FtsK and SpoIIIE: the tale of the conserved tails. Mol Microbiol. 2007;66:1051–1055. - PubMed
- Bigot S, Saleh O, Cornet F, Allemand J, Barre F. Oriented loading of FtsK on KOPS. Nat Struct Mol Biol. 2006;13:1026–1028. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 GM080864/GM/NIGMS NIH HHS/United States
- GM074739/GM/NIGMS NIH HHS/United States
- R01 GM082848/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 GM074739/GM/NIGMS NIH HHS/United States
- WT083469MA/WT_/Wellcome Trust/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- F32GM80864/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases