Surveillance, phagocytosis, and inflammation: how never-resting microglia influence adult hippocampal neurogenesis - PubMed (original) (raw)
Review
Surveillance, phagocytosis, and inflammation: how never-resting microglia influence adult hippocampal neurogenesis
Amanda Sierra et al. Neural Plast. 2014.
Abstract
Microglia cells are the major orchestrator of the brain inflammatory response. As such, they are traditionally studied in various contexts of trauma, injury, and disease, where they are well-known for regulating a wide range of physiological processes by their release of proinflammatory cytokines, reactive oxygen species, and trophic factors, among other crucial mediators. In the last few years, however, this classical view of microglia was challenged by a series of discoveries showing their active and positive contribution to normal brain functions. In light of these discoveries, surveillant microglia are now emerging as an important effector of cellular plasticity in the healthy brain, alongside astrocytes and other types of inflammatory cells. Here, we will review the roles of microglia in adult hippocampal neurogenesis and their regulation by inflammation during chronic stress, aging, and neurodegenerative diseases, with a particular emphasis on their underlying molecular mechanisms and their functional consequences for learning and memory.
Figures
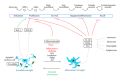
Figure 1
The effects of surveillant and inflammatory microglia on the adult hippocampal neurogenic cascade. During physiological conditions, surveillant microglia effectively phagocytose the excess of apoptotic newborn cells and may release antineurogenic factors such as TGF_β_. This anti-inflammatory state is maintained by neuronal (tethered or released) fractalkine. Enriched environment drives microglia towards a phenotype supportive of neurogenesis, via the production of IGF-1. In contrast, inflammatory challenge triggered by LPS, irradiation, aging, or AD induces the production of proinflammatory cytokines such as IL-1_β_, TNF_α_, and IL-6 by microglia as well as resident astrocytes and infiltrating monocytes, neutrophils, and lymphocytes. These cytokines have profound detrimental effects on adult neurogenesis by reducing the proliferation, survival, integration, and differentiation of the newborn neurons and decreasing their recall during learning and memory paradigms.
Similar articles
- Microglia Actively Remodel Adult Hippocampal Neurogenesis through the Phagocytosis Secretome.
Diaz-Aparicio I, Paris I, Sierra-Torre V, Plaza-Zabala A, Rodríguez-Iglesias N, Márquez-Ropero M, Beccari S, Huguet P, Abiega O, Alberdi E, Matute C, Bernales I, Schulz A, Otrokocsi L, Sperlagh B, Happonen KE, Lemke G, Maletic-Savatic M, Valero J, Sierra A. Diaz-Aparicio I, et al. J Neurosci. 2020 Feb 12;40(7):1453-1482. doi: 10.1523/JNEUROSCI.0993-19.2019. Epub 2020 Jan 2. J Neurosci. 2020. PMID: 31896673 Free PMC article. - Lifestyle Shapes the Dialogue between Environment, Microglia, and Adult Neurogenesis.
Valero J, Paris I, Sierra A. Valero J, et al. ACS Chem Neurosci. 2016 Apr 20;7(4):442-53. doi: 10.1021/acschemneuro.6b00009. Epub 2016 Mar 22. ACS Chem Neurosci. 2016. PMID: 26971802 Review. - Neurogenesis, inflammation and behavior.
Kohman RA, Rhodes JS. Kohman RA, et al. Brain Behav Immun. 2013 Jan;27(1):22-32. doi: 10.1016/j.bbi.2012.09.003. Epub 2012 Sep 15. Brain Behav Immun. 2013. PMID: 22985767 Free PMC article. Review. - Impact of neurodegenerative diseases on human adult hippocampal neurogenesis.
Terreros-Roncal J, Moreno-Jiménez EP, Flor-García M, Rodríguez-Moreno CB, Trinchero MF, Cafini F, Rábano A, Llorens-Martín M. Terreros-Roncal J, et al. Science. 2021 Nov 26;374(6571):1106-1113. doi: 10.1126/science.abl5163. Epub 2021 Oct 21. Science. 2021. PMID: 34672693 Free PMC article. - Role of Microglia in Modulating Adult Neurogenesis in Health and Neurodegeneration.
Al-Onaizi M, Al-Khalifah A, Qasem D, ElAli A. Al-Onaizi M, et al. Int J Mol Sci. 2020 Sep 19;21(18):6875. doi: 10.3390/ijms21186875. Int J Mol Sci. 2020. PMID: 32961703 Free PMC article. Review.
Cited by
- Erinacine A-enriched Hericium erinaceus mycelium ameliorates Alzheimer's disease-related pathologies in APPswe/PS1dE9 transgenic mice.
Tsai-Teng T, Chin-Chu C, Li-Ya L, Wan-Ping C, Chung-Kuang L, Chien-Chang S, Chi-Ying HF, Chien-Chih C, Shiao YJ. Tsai-Teng T, et al. J Biomed Sci. 2016 Jun 27;23(1):49. doi: 10.1186/s12929-016-0266-z. J Biomed Sci. 2016. PMID: 27350344 Free PMC article. - Rewiring of Memory Circuits: Connecting Adult Newborn Neurons With the Help of Microglia.
Rodríguez-Iglesias N, Sierra A, Valero J. Rodríguez-Iglesias N, et al. Front Cell Dev Biol. 2019 Mar 5;7:24. doi: 10.3389/fcell.2019.00024. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 30891446 Free PMC article. Review. - Inflammatory signaling pathways in the treatment of Alzheimer's disease with inhibitors, natural products and metabolites (Review).
Zheng Y, Zhang X, Zhang R, Wang Z, Gan J, Gao Q, Yang L, Xu P, Jiang X. Zheng Y, et al. Int J Mol Med. 2023 Nov;52(5):111. doi: 10.3892/ijmm.2023.5314. Epub 2023 Oct 6. Int J Mol Med. 2023. PMID: 37800614 Free PMC article. Review. - Do changes in microglial status underlie neurogenesis impairments and depressive-like behaviours induced by psychological stress? A systematic review in animal models.
Nieto-Quero A, Chaves-Peña P, Santín LJ, Pérez-Martín M, Pedraza C. Nieto-Quero A, et al. Neurobiol Stress. 2021 Jun 18;15:100356. doi: 10.1016/j.ynstr.2021.100356. eCollection 2021 Nov. Neurobiol Stress. 2021. PMID: 34355047 Free PMC article. Review. - Age-Associated Resident Memory CD8 T Cells in the Central Nervous System Are Primed To Potentiate Inflammation after Ischemic Brain Injury.
Ritzel RM, Crapser J, Patel AR, Verma R, Grenier JM, Chauhan A, Jellison ER, McCullough LD. Ritzel RM, et al. J Immunol. 2016 Apr 15;196(8):3318-30. doi: 10.4049/jimmunol.1502021. Epub 2016 Mar 9. J Immunol. 2016. PMID: 26962232 Free PMC article.
References
- Rezaie P, Male D. Mesoglia and microglia—a historical review of the concept of mononuclear phagocytes within the central nervous system. Journal of the History of the Neurosciences. 2002;11(4):325–374. - PubMed
- Gomez Perdiguero E, Schulz C, Geissmann F. Development and homeostasis of “resident” myeloid cells: the case of the microglia. GLIA. 2013;61(1):112–120. - PubMed
- Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77(1):10–18. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials