Microfluidic single-cell whole-transcriptome sequencing - PubMed (original) (raw)
Microfluidic single-cell whole-transcriptome sequencing
Aaron M Streets et al. Proc Natl Acad Sci U S A. 2014.
Abstract
Single-cell whole-transcriptome analysis is a powerful tool for quantifying gene expression heterogeneity in populations of cells. Many techniques have, thus, been recently developed to perform transcriptome sequencing (RNA-Seq) on individual cells. To probe subtle biological variation between samples with limiting amounts of RNA, more precise and sensitive methods are still required. We adapted a previously developed strategy for single-cell RNA-Seq that has shown promise for superior sensitivity and implemented the chemistry in a microfluidic platform for single-cell whole-transcriptome analysis. In this approach, single cells are captured and lysed in a microfluidic device, where mRNAs with poly(A) tails are reverse-transcribed into cDNA. Double-stranded cDNA is then collected and sequenced using a next generation sequencing platform. We prepared 94 libraries consisting of single mouse embryonic cells and technical replicates of extracted RNA and thoroughly characterized the performance of this technology. Microfluidic implementation increased mRNA detection sensitivity as well as improved measurement precision compared with tube-based protocols. With 0.2 M reads per cell, we were able to reconstruct a majority of the bulk transcriptome with 10 single cells. We also quantified variation between and within different types of mouse embryonic cells and found that enhanced measurement precision, detection sensitivity, and experimental throughput aided the distinction between biological variability and technical noise. With this work, we validated the advantages of an early approach to single-cell RNA-Seq and showed that the benefits of combining microfluidic technology with high-throughput sequencing will be valuable for large-scale efforts in single-cell transcriptome analysis.
Keywords: embryonic stem cell; genomics; lab on chip.
Figures
Fig. 1.
Device schematic and experimental pipeline. (A) Micrograph of the microfluidic device filled with colored dye. Blue lines are the control channels, and purple lines are the flow channels. The single-cell suspension was injected into cell input, and reagents were injected into reagent input. Double-stranded cDNA was recovered from the output ports. (B) Detailed diagram of a single-reaction pipeline. After a single cell was trapped in the trapping chamber (0.86 nL), it was pushed into the sorting chamber (S; 1.35 nL) and then, consecutive reactions for cell lysis (1; 3.82 nL), reverse transcription (2; 3.82 nL), polyA tailing (3; 2.70 nL), primer digestion (4; 10.1 nL), and second-strand cDNA synthesis (5; 128 nL). (C) Complete experimental pipeline. Off-chip amplification and library preparation are explained in
SI Materials and Methods
.
Fig. 2.
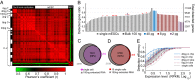
Single-cell transcriptome sequencing sensitivity. (A) Pairwise Pearson correlation coefficient between expression levels of all genes with RPKM > 1 for each library (80 in total). Discarded cells and negative control libraries were excluded (
Table S1
). The left vertical and top horizontal axes labels identify library type. T represents tube experiments, and C represents chip experiments. Black and white in the left vertical axis denote cDNA prepared in separate microfluidic devices. (B) Gene detection in 48 single-cell and 23 technical replicate libraries ranked by their total number of detected genes and compared with 100-ng bulk extracted RNA. The single-cell data are plotted with sample name labels as in
Fig. S4_C_
. (C) Comparison of genes detected with RPKM > 1 in a typical cell (indicated with a purple arrow in B) with the genes detected in the 100-ng bulk sample. All genes within the purple circle were detected in at least 2 of 48 single cells. The percent is the ratio of the overlapping region to the entire bulk circle. (D) Genes detected in 10 randomly selected cells (indicated with red arrows in B) after randomly sampling 200,000 reads from each library and mapping them to the reference sequence (red circle) compared with the genes detected in 2 million reads from the 100-ng bulk sample (gray circle). (E) The ratio of genes detected in the single-cell libraries (gray lines) and technical replicate libraries to the genes detected in the bulk library binned by expression level. Error bars indicate SD in the following technical replicates: solid blue line, 40 pg in chip (n = 3); dashed blue line, 40 pg in tube (n = 3); solid red line, 8 pg in chip (n = 16); dashed red line, 8 pg in tube (n = 3).
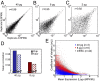
Fig. 3.
Assessment of microfluidic single-cell RNA-Seq reproducibility. Scatter plots show the correlation between technical replicates of extracted mESC RNA diluted to (A) 40 pg (B40-2 and B40-3), (B) 8 pg (B8-12 and B8-15), and (C) 2 pg (B2-1 and B2-2) with the Pearson correlation coefficient (r). (D) For each extracted RNA dilution, the correlation was measured for all pairs of replicates. The mean of these correlation coefficients is displayed for 40 pg (blue) and 8 pg (red) in both chip (solid bars) and tube (striped bars). The number of replicates for each dataset is the same as in Fig. 2_E_. (E) The CV (SD normalized by the mean) is plotted against the log10-transformed geometric mean of expression for all genes detected in 44 single mESCs (gray dots), 16 8-pg technical replicates (red dots), and 3 40-pg technical replicates (blue dots). The spread in variation for the extracted RNA samples represents technical noise, whereas the variation in single-cell expression is a combination of technical and biological variation.
Fig. 4.
Assessment of accuracy with RNA spike-in. (A) Mean expression of ERCC spike across three samples: mESC42 (negative control), mESC43 (negative control), and mESC44 (discarded cell). Samples were chosen because of their large number and high ratio of reads mapped to the ERCC reference (
Fig. S6 A and B
). (B) Detection of Cre spike-in abundance in 27 samples that each contained two Cre molecules on average.
Fig. 5.
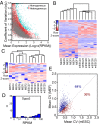
Variation in gene expression within and between mESCs and MEFs. (A) The CV in genes expressed in a group of 12 single mESCs (gray dots) and a group of 6 mESCs and 6 MEFs (red dots) plotted against mean expression. Genes in the heterogeneous population exhibiting a CV greater than 3 SDs above the mean CV of 12 mESCs were considered to show high variability and are colored turquoise (
Dataset S1
). (B) Unsupervised hierarchical clustering of six mESCs and six MEFs based on the expression levels of 689 genes found to show high variation between the two cell types in A. (C) Unsupervised hierarchical clustering of six mESCs and six MEFs based on expression levels of 38 long noncoding RNAs that showed high variability between the two cell types (
Fig. S6_C_
and
Dataset S1
). (D) Histogram of expression levels of Dppa3 in 44 single mESC libraries. (E) Correlation of CV between genes expressed with RPKM > 50 in six MEF cells and six typical mESCs (labeled purple in
Fig. S4_C_
). The percentages represent the fraction of genes with higher CV among MEF cells than mESCs (blue) and lower CV among MEF cells than mESCs (red). Similar plots with various sets of six mESCs are shown in
Fig. S7
.
Similar articles
- Single-Cell mRNA-Seq Using the Fluidigm C1 System and Integrated Fluidics Circuits.
Gong H, Do D, Ramakrishnan R. Gong H, et al. Methods Mol Biol. 2018;1783:193-207. doi: 10.1007/978-1-4939-7834-2_10. Methods Mol Biol. 2018. PMID: 29767364 - Recovery and analysis of transcriptome subsets from pooled single-cell RNA-seq libraries.
Riemondy KA, Ransom M, Alderman C, Gillen AE, Fu R, Finlay-Schultz J, Kirkpatrick GD, Di Paola J, Kabos P, Sartorius CA, Hesselberth JR. Riemondy KA, et al. Nucleic Acids Res. 2019 Feb 28;47(4):e20. doi: 10.1093/nar/gky1204. Nucleic Acids Res. 2019. PMID: 30496484 Free PMC article. - Single-Cell Transcriptomics of Immune Cells: Cell Isolation and cDNA Library Generation for scRNA-Seq.
Arsenio J. Arsenio J. Methods Mol Biol. 2020;2184:1-18. doi: 10.1007/978-1-0716-0802-9_1. Methods Mol Biol. 2020. PMID: 32808214 - Current and Future Methods for mRNA Analysis: A Drive Toward Single Molecule Sequencing.
Bayega A, Fahiminiya S, Oikonomopoulos S, Ragoussis J. Bayega A, et al. Methods Mol Biol. 2018;1783:209-241. doi: 10.1007/978-1-4939-7834-2_11. Methods Mol Biol. 2018. PMID: 29767365 Review. - Preparation of Single-Cell RNA-Seq Libraries for Next Generation Sequencing.
Trombetta JJ, Gennert D, Lu D, Satija R, Shalek AK, Regev A. Trombetta JJ, et al. Curr Protoc Mol Biol. 2014 Jul 1;107:4.22.1-4.22.17. doi: 10.1002/0471142727.mb0422s107. Curr Protoc Mol Biol. 2014. PMID: 24984854 Free PMC article. Review.
Cited by
- Single cell transcriptomics: methods and applications.
Kanter I, Kalisky T. Kanter I, et al. Front Oncol. 2015 Mar 10;5:53. doi: 10.3389/fonc.2015.00053. eCollection 2015. Front Oncol. 2015. PMID: 25806353 Free PMC article. Review. - High-generation near-isogenic lines combined with multi-omics to study the mechanism of polima cytoplasmic male sterility.
Wang B, Farooq Z, Chu L, Liu J, Wang H, Guo J, Tu J, Ma C, Dai C, Wen J, Shen J, Fu T, Yi B. Wang B, et al. BMC Plant Biol. 2021 Mar 5;21(1):130. doi: 10.1186/s12870-021-02852-7. BMC Plant Biol. 2021. PMID: 33673810 Free PMC article. - Viro-fluidics: Real-time analysis of virus production kinetics at the single-cell level.
Eid J, Socol M, Naillon A, Feuillard J, Ciandrini L, Margeat E, Charlot B, Mougel M. Eid J, et al. Biophys Rep (N Y). 2022 Aug 11;2(3):100068. doi: 10.1016/j.bpr.2022.100068. eCollection 2022 Sep 14. Biophys Rep (N Y). 2022. PMID: 36425325 Free PMC article. - Review of methods to probe single cell metabolism and bioenergetics.
Vasdekis AE, Stephanopoulos G. Vasdekis AE, et al. Metab Eng. 2015 Jan;27:115-135. doi: 10.1016/j.ymben.2014.09.007. Epub 2014 Oct 31. Metab Eng. 2015. PMID: 25448400 Free PMC article. Review. - Microfluidics: reframing biological enquiry.
Duncombe TA, Tentori AM, Herr AE. Duncombe TA, et al. Nat Rev Mol Cell Biol. 2015 Sep;16(9):554-67. doi: 10.1038/nrm4041. Nat Rev Mol Cell Biol. 2015. PMID: 26296163 Free PMC article. Review.
References
- Guo G, et al. Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Dev Cell. 2010;18(4):675–685. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous