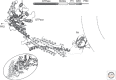Molecular structure, function, and dynamics of clathrin-mediated membrane traffic - PubMed (original) (raw)
Review
Molecular structure, function, and dynamics of clathrin-mediated membrane traffic
Tom Kirchhausen et al. Cold Spring Harb Perspect Biol. 2014.
Abstract
Clathrin is a molecular scaffold for vesicular uptake of cargo at the plasma membrane, where its assembly into cage-like lattices underlies the clathrin-coated pits of classical endocytosis. This review describes the structures of clathrin, major cargo adaptors, and other proteins that participate in forming a clathrin-coated pit, loading its contents, pinching off the membrane as a lattice-enclosed vesicle, and recycling the components. It integrates as much of the structural information as possible at the time of writing into a sketch of the principal steps in coated-pit and coated-vesicle formation.
Figures
Figure 1.
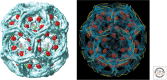
Clathrin. (A) Organization of the heavy- and light-chain polypeptide chains. The name or phase designating various segments appears above the corresponding region of the sequence. Human cells have two light chains (LCa and LCb), each with tissue-specific splice variants, yielding the range of lengths shown. There is no detectable preference of one or the other for association with a heavy chain; the central, α-helical segment, which mediates heavy-chain association, is almost completely conserved in all light-chain forms. A second heavy-chain gene in human cells encodes a paralog found in muscle and fat and involved in intracellular traffic, but not in endocytosis; it does not bind light chains. (B, left) Full α-carbon (Cα) representation of a clathrin triskelion, viewed along its threefold axis as if from the outside of a coat. The various segments of the heavy chain are labeled, and the central α-helical region of the light chain on each leg is also shown. (C) Packing of triskelions in a clathrin coat. The structure shown is that of a “D6 barrel,” one of the simplest and smallest coated-vesicle lattices. Each triskelion is drawn as a “worm” extending from the hook-like representation of the terminal domain on the inside of the shell to the vertex of the triskelion at the outside. (Blue) One triskelion, in an orientation similar to the one in B. (D, left) Side view of the triskelion, in the same representation and scale as in B. (Right) Blowup of the part of a triskelion close to the threefold axis, including the tripod and the disordered, carboxy-terminal segment with a site (QLMLT) for Hsc70 binding. (From Xing et al. 2010; adapted, with permission, from the authors.)
Figure 2.
A D6 clathrin coat. (A) Map from electron microscopy of a coat with bound auxilin (clathrin-binding and J-domain fragment) and Hsc70, showing the outer contour of clathrin density (blue), auxilin (red), and Hsc70 (green). (From Xing et al. 2010; with permission from the authors.) (B) Full Cα representation of the heavy chains in a coat (blue), with ribbon representation of light chain σ-helical region (yellow) and volume outline of auxilin clathrin-binding and J-domain fragment (red) (Fotin et al. 2004a,b).
Figure 3.
Coated vesicles, isolated from brain and examined by electron cryotomography. (Top) Gallery of central sections through tomograms of individual coated vesicles. (Bottom) Drawing of corresponding lattices, with position of vesicle shown (Cheng et al. 2007).
Figure 4.
Heterotetrameric clathrin adaptor, AP2. (A) Domain organization of polypeptide chains of the four component proteins. The two heavy chains, α and β2, have amino-terminal HEAT-repeat domains connected by a flexible hinge (dashed line) to a carboxy-terminal appendage domain; their total length is about 940 amino acid residues. The arrow in the β2 diagram shows the approximate position of the clathrin-box motif that binds the clathrin terminal domain. The lengths of the bars are in approximate proportion to the number of residues in the corresponding domain. (B) Ribbon representation of the complete adaptor in its “locked” state. The flexible hinge regions of the heavy chains are shown as dotted lines. Colors as in A. (PDB: 2VGL, 1B9K, 1E42) (C–E) Ribbon representations of the AP2 “core” comprising the HEAT-repeat domains of the heavy chains and the complete µ and σ chains. The “locked” (PDB 2VGL) (Collins et al. 2002), “unlatched” (PDB:2JKR) (Kelly et al. 2008), and “open” (PDB:2XA7) (Jackson et al. 2010) conformations are in C, D, and E, respectively. Arrows in D show directions in which the heat-domain domains bend (bend concentrated mainly at the elbow) to achieve the open conformation, in which the μC domain also rotates substantially. (F–H) Diagrams corresponding to the molecular representations above them. (Gray bar) Membrane, with PtdIns(4,5)P2 (schematic). (Circles on heavy chains) Sites for PtdIns(4,5)P2 headgroup binding. Yellow “v” in G and H points to the position of the site for dileucine motif binding; (red asterisk on µ2-C domain) position of site for tyrosine-motif binding. Note that lipid-headgroup and cargo-recognition sites all line up near the membrane in this conformation.
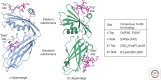
Figure 5.
AP2 appendages. Ribbon representations of α (blue) and β2 (green) appendages with top site and side site cognate peptides (magenta stick representation). The motifs recognized are shown in the table to the right.
Figure 6.
Adaptors and accessory proteins. A domain diagram of the full protein above the molecular structure shows the relationship of the domain illustrated to the complete polypeptide chain. (A) Disheveled (Dvl) DEP domain and tyrosine motif, bound to µ2-C (PDB 3ML6) (Yu et al. 2010), superposed onto the structure of the open AP2 core (PDB 2XA7) (Jackson et al. 2010). The tyrosine motif is represented as sticks; the DEP domain (dark gray) and µ2-C (lighter gray) as ribbons; the rest of the AP2 core as a surface. (B) CALM/AP180 ANTH domain, modeled bound with the PtdIns(4,5)P2 head group (PDB 1HG2) (Ford et al. 2001), and its complex with peptide from VAMP (PDB 3GYM) (Miller et al. 2011). (C) Autosomal recessive hypercholesterolemia (ARH) phosphotyrosine-binding (PTB) domain (ribbons) bound with peptide (sticks) from cytoplasmic tail of LDL receptor and modeled with the PtdIns(4,5)P2 head group (PDB 3SO6) (Dvir et al. 2012). (D) Epsin ENTH domain with the PtdIns(4,5)P2 head group (PDB 1H0A) (Ford et al. 2002). (E) FCHo F-BAR domain and μ-homology domain (MHD); structure of the former from FCHo2 (PDB 2V0O) (Henne et al. 2007) and of the latter from the yeast homolog Syp1 (PDB 3G9H) (Reider et al. 2009). The F-BAR domain binds preferentially to a curved membrane bilayer.
Figure 7.
Dynamin. Domain diagram shows position in polypeptide chain of GTPase domain (somewhat augmented at both ends from the canonical small GTPase), “middle” domain, PH domain, GTPase effector domain (GED), and proline-rich domain (PRD). The large ribbon diagram shows a dynamin monomer, with PRD at the carboxyl terminus removed (PDB 3ZVR) (Ford et al. 2011). Note that the carboxy-terminal part of the GED region abuts the GTPase domain and influences its conformation. The PH domain interacts with the constricted membrane (dashed lines) at the neck of a budding coat. (Inset) GTP binding induces dimerization of the GTPase domains of two dynamins and switches the relative orientations of GTPase and GED. Two GMPPCP molecules bound at the dimer interface are shown as atomic models (spheres) (PDB 3ZYC) (Chappie et al. 2011). The orientation of one of the two GTPase domains (light gray) is the same as in the figure of the complete molecule; there distributed conformational changes, in addition to dimer formation, that accompany GTP binding.
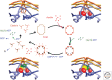
Figure 8.
Assembly and disassembly of a canonical, endocytic clathrin-coated pit. AP2 adaptor complexes, associated at the membrane with PtdIns(4,5)P2 (PIP2), recruit clathin triskelions to initiate lattice assembly. Stable growth and lattice closure require endocytic accessory proteins (Eps15, epsin, FCHo1/2, intersectin, CALM/AP180; some of which are also ancillary cargo adaptors). Dynamin, assisted by actin polymerization when the membrane is under tension, drives membrane scission and coated-vesicle release. Hsc70, recruited by the J-domain protein auxilin, mediates clathrin uncoating and release of a free vesicle, primed to fuse with a target membrane. Text beneath the diagram indicates the overall timescale and the stages at which various components appear to function. Short arcs, Clathrin triskelions; T shapes, AP2.
Figure 9.
Hsc70-mediated uncoating. Illustration of proposed mechanism. (Upper left) Vertex of a clathrin lattice, with clathrin polypeptide chains as continuous worms. (Orange) The hub of a triskelion centered on that vertex; with the carboxy-terminal Hsc-70-binding segment dotted; (yellow) the “knees” of triskelions centered on the neighboring vertices; (blue) the terminal domains and linkers of triskelions centered on second-nearest-neighbor vertices. See Figure 1 to place this vertex in the context of a full lattice. (Upper right) Binding of the carboxy-terminal region of auxilin, containing the clathrin-binding segment and the J-domain; the amino-terminal region, which contains the PTEN-homology domain, would project inward toward the vesicle membrane. (Lower right) Auxilin J-domain recruits Hsc70:ATP. (Lower left) Hsc70, in a conformational change driven by ATP hydrolysis, clamps tightly onto its recognition motif on one of the three clathrin carboxy-terminal segments. This event locks in a local distortion, necessary to expose the recognition motif enough to accommodate the Hsc70 clamp domain. Binding of Hsc70 at a critical number of vertices imposes sufficient distortion to destabilize the entire lattice. Rebinding of ATP to Hsc70 dissociates it from the free triskelions, which can then assemble into a new coat and complete the cycle. (From Xing et al. 2010 and Böcking et al. 2011; adapted, with permission, from the authors.)
Similar articles
- Distinct dynamics of endocytic clathrin-coated pits and coated plaques.
Saffarian S, Cocucci E, Kirchhausen T. Saffarian S, et al. PLoS Biol. 2009 Sep;7(9):e1000191. doi: 10.1371/journal.pbio.1000191. Epub 2009 Sep 8. PLoS Biol. 2009. PMID: 19809571 Free PMC article. - Physical and functional connection between auxilin and dynamin during endocytosis.
Sever S, Skoch J, Newmyer S, Ramachandran R, Ko D, McKee M, Bouley R, Ausiello D, Hyman BT, Bacskai BJ. Sever S, et al. EMBO J. 2006 Sep 20;25(18):4163-74. doi: 10.1038/sj.emboj.7601298. Epub 2006 Aug 31. EMBO J. 2006. PMID: 16946707 Free PMC article. - Cortactin is a component of clathrin-coated pits and participates in receptor-mediated endocytosis.
Cao H, Orth JD, Chen J, Weller SG, Heuser JE, McNiven MA. Cao H, et al. Mol Cell Biol. 2003 Mar;23(6):2162-70. doi: 10.1128/MCB.23.6.2162-2170.2003. Mol Cell Biol. 2003. PMID: 12612086 Free PMC article. - Clathrin-coated vesicle formation and protein sorting: an integrated process.
Schmid SL. Schmid SL. Annu Rev Biochem. 1997;66:511-48. doi: 10.1146/annurev.biochem.66.1.511. Annu Rev Biochem. 1997. PMID: 9242916 Review. - The structural era of endocytosis.
Marsh M, McMahon HT. Marsh M, et al. Science. 1999 Jul 9;285(5425):215-20. doi: 10.1126/science.285.5425.215. Science. 1999. PMID: 10398591 Review.
Cited by
- Exploring the role of riboflavin in swine well-being: a literature review.
Shastak Y, Pelletier W. Shastak Y, et al. Porcine Health Manag. 2024 Oct 31;10(1):46. doi: 10.1186/s40813-024-00399-1. Porcine Health Manag. 2024. PMID: 39482748 Free PMC article. Review. - Activity-Regulated Cytoskeleton-Associated Protein Controls AMPAR Endocytosis through a Direct Interaction with Clathrin-Adaptor Protein 2.
DaSilva LL, Wall MJ, P de Almeida L, Wauters SC, Januário YC, Müller J, Corrêa SA. DaSilva LL, et al. eNeuro. 2016 May 24;3(3):ENEURO.0144-15.2016. doi: 10.1523/ENEURO.0144-15.2016. eCollection 2016 May-Jun. eNeuro. 2016. PMID: 27257628 Free PMC article. - FluoSTEPs: Fluorescent biosensors for monitoring compartmentalized signaling within endogenous microdomains.
Tenner B, Zhang JZ, Kwon Y, Pessino V, Feng S, Huang B, Mehta S, Zhang J. Tenner B, et al. Sci Adv. 2021 May 21;7(21):eabe4091. doi: 10.1126/sciadv.abe4091. Print 2021 May. Sci Adv. 2021. PMID: 34020947 Free PMC article. - Transcellular routes of blood-brain barrier disruption.
Erickson MA, Banks WA. Erickson MA, et al. Exp Biol Med (Maywood). 2022 May;247(9):788-796. doi: 10.1177/15353702221080745. Epub 2022 Mar 4. Exp Biol Med (Maywood). 2022. PMID: 35243912 Free PMC article. - The GTPase Arf1 Is a Determinant of Yeast Vps13 Localization to the Golgi Apparatus.
Kolakowski D, Rzepnikowska W, Kaniak-Golik A, Zoladek T, Kaminska J. Kolakowski D, et al. Int J Mol Sci. 2021 Nov 12;22(22):12274. doi: 10.3390/ijms222212274. Int J Mol Sci. 2021. PMID: 34830155 Free PMC article.
References
- Anderson RG, Goldstein JL, Brown MS 1977. A mutation that impairs the ability of lipoprotein receptors to localise in coated pits on the cell surface of human fibroblasts. Nature 270: 695–699 - PubMed
- Bao H, Daniels RW, MacLeod GT, Charlton MP, Atwood HL, Zhang B 2005. AP180 maintains the distribution of synaptic and vesicle proteins in the nerve terminal and indirectly regulates the efficacy of Ca2+-triggered exocytosis. J Neurophysiol 94: 1888–1903 - PubMed
- Bennett EM, Chen CY, Engqvist-Goldstein AE, Drubin DG, Brodsky FM 2001. Clathrin hub expression dissociates the actin-binding protein Hip1R from coated pits and disrupts their alignment with the actin cytoskeleton. Traffic 2: 851–858 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases