DNA methylation in mammals - PubMed (original) (raw)
Review
DNA methylation in mammals
En Li et al. Cold Spring Harb Perspect Biol. 2014.
Abstract
DNA methylation is one of the best characterized epigenetic modifications. In mammals it is involved in various biological processes including the silencing of transposable elements, regulation of gene expression, genomic imprinting, and X-chromosome inactivation. This article describes how DNA methylation serves as a cellular memory system and how it is dynamically regulated through the action of the DNA methyltransferase (DNMT) and ten eleven translocation (TET) enzymes. Its role in the regulation of gene expression, through its interplay with histone modifications, is also described, and its implication in human diseases discussed. The exciting areas of investigation that will likely become the focus of research in the coming years are outlined in the summary.
Figures
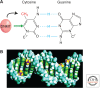
Figure 1.
Cytosine methylation in DNA. (A) Addition of a methyl group, CH3 (red), at the five position of the cytosine pyrimidine ring (black arrow) does not sterically interfere with GC base pairing (blue lines). DNA methyltransferases associate covalently with the carbon 6 position (straight green arrow) during methyl group transfer. (B) A model of B-form DNA methylated at cytosines in two self-complementary CpG sequences. The paired methyl moieties (magenta and yellow) lie in the major groove of the double helix.
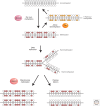
Figure 2.
De novo methylation and maintenance methylation of DNA. A stretch of genomic DNA is shown as a line with self-complementary CpG pairs marked as vertical strokes. Unmethylated DNA (top) becomes methylated “de novo” by Dnmt3a and Dnmt3b to give symmetrical methylation at certain CpG pairs. On semiconservative DNA replication, a progeny DNA strand is base-paired with one of the methylated parental strands (the other replication product is not shown). Symmetry is restored by the maintenance DNA methyltransferase, Dnmt1, which completes half-methylated sites, but does not methylate unmodified CpGs.
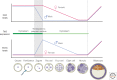
Figure 3.
Dynamics of 5mC/5hmC/5fC/5caC in paternal and maternal genomes during preimplantation development. DNA demethylation of the zygote, gauged by 5mC levels, occurs by a passive mechanism in the female pronucleus, diluting the marks with the passage of every cell cycle. The male pronuclear genome becomes demethylated actively by the action of the Tet enzymes. Tet3 is expressed in the oocyte and zygote. After fertilization, Tet3 is relocated from the cytoplasm to the paternal nucleus to convert 5mC to 5hmC/5fC/5caC. Subsequently, paternal and maternal genomes undergo replication-dependent dilution of 5hmC/5fC/5caC in males and 5mC in females. It is possible that replication-independent active DNA demethylation may occur in a loci-specific manner in zygotes, but the exact mechanism is currently unclear. DNA methylation patterns in ICM are reestablished by de novo DNA methyltransferases Dnmt3a and Dnmt3b at the blastocyst stage.
Figure 4.
Mammalian DNA methyltransferases. The catalytic domains of Dnmt1, Dnmt2, and the Dnmt3 family members are conserved (the signature motifs, I, IV, VI, IX, and X, are most conserved in all cytosine methyltransferases), but there is little similarity among their amino-terminal regulatory domains. Domain abbreviations: PCNA, PCNA-interacting domain; NLS, nuclear localization signal; RFT, replication foci-targeting domain; CXXC, a cysteine-rich domain implicated in binding DNA sequences containing CpG dinucleotides; BAH, bromo-adjacent homology domain implicated in protein–protein interactions; PWWP, a domain containing a highly conserved “proline-tryptophan-tryptophan-proline” motif involved in heterochromatin association; ATRX, an ATRX-related cysteine-rich region containing a C2-C2 zinc finger and an atypical PHD domain implicated in protein–protein interactions.
Figure 5.
CpG islands. (A) CGIs are regions of high CpG density (>50%), usually 200 bp–2 kb in length that lack CpG methylation, found at promoters of most human genes. Long-term silencing of the gene can be insured by methylation of the CGI region. For example, genes on the inactive X chromosome and certain imprinted genes are silenced in this way. Also, in cancer cells certain genes are aberrantly silenced by CGI methylation. Shores are regions of the genome that reside up to 2 kb from CGIs, whereas shelves are found 2–4 kb away from CGIs. (B) Chromatin immunoprecipation (IP) analysis of Kdm2b binding sites shows that Kdm2b is enriched at the CpGs of the Hox locus in which the unmethylated CGIs (green bars) are located. (C) Cfp1, Kdm2a, and Kdm2b proteins share a common CXXC domain that binds specifically to unmethylated CpG sites. Protein length is indicated to the right of each protein. Abbreviations for other domains include PHD, plant homeodomain; A, acidic domain; B, basic domain; S, Set1 interacting domain; C, coiled coil domain; LRR, leucine-rich repeat domain.
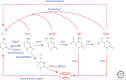
Figure 6.
Model of Tet-initiated DNA demethylation pathways. DNA methylation (5mC) is established and maintained by DNMT. 5mC can by oxidized by Tet family of dioxygenases to generate 5hmC, 5fC, and 5caC. Because the oxidized 5mC derivatives cannot serve as substrates for DNMT1, they can be lost by replication-dependent passive demethylation. 5hmC can be deaminated by AID/APOBEC to become 5hmU, which together with 5fC and 5caC can be excised by glycosylases such as TDG, followed by DNA repair to generate C. Alternatively, a putative decarboxylase may convert 5caC to C.
Figure 7.
Domain structure of the mouse Tet family proteins. Schematic diagrams of predicted conserved domain structures in the three mouse Tet proteins. The conserved domains include CXXC zinc-binding domain, the cysteine-rich domain, and the double-stranded β-helix (DSBH) found in members of the dioxygenase superfamily proteins. Both the Cys-rich and the DSBH domains have been shown to be critical for enzymatic activity. Numbers indicate amino acid numbers.
Figure 8.
Proteins that bind methyl-CpG. Five members of the MBD protein family are aligned at their MBD domains (purple). Other domains are labeled and include TRD; CXXC domains, which are zinc fingers, some of which are implicated in binding to nonmethylated CpG; GR repeats that may bind; a T:G mismatch glycosylase domain that is involved in repair of 5mC deamination. Kaiso lacks the MBD domain, but binds methylated DNA via zinc fingers (orange) and possesses a POB/BTB domain that is shared with other transcriptional repressors. Domain abbreviations: MBD, methyl-CpG binding domain; TRD, transcriptional repression domain; POZ, poxvirus and zinc finger, a protein–protein interacting domain.
Figure 9.
Recruitment of corepressors by methyl-CpG binding proteins. A hypothetical transition between an active, nonmethylated gene promoter and a repressed promoter whose silence is attributable to DNA methylation, as mediated by complexes containing an MBD protein such as MeCP2 (gray shading). The transition phase represents an intermediate step during which transcription is silenced and DNA methylation occurs. MeCP2 is envisaged to recruit the NCoR histone deacetylase (HDAC) complex and histone lysine methyltransferase (HKMT) activity to the methylated sites. In addition, there is some evidence that MeCP2 can directly repress (DR) transcription by contact with the transcription initiation complex. Other methyl-CpG binding proteins can also interact with and potentially recruit distinct corepressor complexes that include HKMT and/or HDAC activity. PRC1 and PRC2 are also involved in silencing gene expression through histone H3K27 methylation, catalyzed by PRC2 (left outcome). One of the mechanisms by which they function together to regulate the same set of target genes is through the recognition of the H3K27me3 mark by the chromodomain protein in the PRC1 complex. PRC1 can also be recruited by the CxxC domain–containing protein Kdm2b to effect gene silencing (right outcome). Proteins with known histone posttranslational modifying (PTM) activity are indicated.
Similar articles
- Regulation of CpG methylation by Dnmt and Tet in pluripotent stem cells.
Horii T, Hatada I. Horii T, et al. J Reprod Dev. 2016 Aug 25;62(4):331-5. doi: 10.1262/jrd.2016-046. Epub 2016 May 5. J Reprod Dev. 2016. PMID: 27151232 Free PMC article. Review. - Epstein-barr virus latency in B cells leads to epigenetic repression and CpG methylation of the tumour suppressor gene Bim.
Paschos K, Smith P, Anderton E, Middeldorp JM, White RE, Allday MJ. Paschos K, et al. PLoS Pathog. 2009 Jun;5(6):e1000492. doi: 10.1371/journal.ppat.1000492. Epub 2009 Jun 26. PLoS Pathog. 2009. PMID: 19557159 Free PMC article. - DNA methyltransferase 1 knock down induces gene expression by a mechanism independent of DNA methylation and histone deacetylation.
Milutinovic S, Brown SE, Zhuang Q, Szyf M. Milutinovic S, et al. J Biol Chem. 2004 Jul 2;279(27):27915-27. doi: 10.1074/jbc.M312823200. Epub 2004 Apr 15. J Biol Chem. 2004. PMID: 15087453 - The "Epigenetic Code Replication Machinery", ECREM: a promising drugable target of the epigenetic cell memory.
Bronner C, Chataigneau T, Schini-Kerth VB, Landry Y. Bronner C, et al. Curr Med Chem. 2007;14(25):2629-41. doi: 10.2174/092986707782023244. Curr Med Chem. 2007. PMID: 17979715 Review. - Epigenetic response to environmental stress: Assembly of BRG1-G9a/GLP-DNMT3 repressive chromatin complex on Myh6 promoter in pathologically stressed hearts.
Han P, Li W, Yang J, Shang C, Lin CH, Cheng W, Hang CT, Cheng HL, Chen CH, Wong J, Xiong Y, Zhao M, Drakos SG, Ghetti A, Li DY, Bernstein D, Chen HS, Quertermous T, Chang CP. Han P, et al. Biochim Biophys Acta. 2016 Jul;1863(7 Pt B):1772-81. doi: 10.1016/j.bbamcr.2016.03.002. Epub 2016 Mar 4. Biochim Biophys Acta. 2016. PMID: 26952936 Free PMC article.
Cited by
- Transcriptional Silencers: Driving Gene Expression with the Brakes On.
Segert JA, Gisselbrecht SS, Bulyk ML. Segert JA, et al. Trends Genet. 2021 Jun;37(6):514-527. doi: 10.1016/j.tig.2021.02.002. Epub 2021 Mar 9. Trends Genet. 2021. PMID: 33712326 Free PMC article. Review. - Uncovering epigenetic landscape: a new path for biomarkers identification and drug development.
de Oliveira DT, Guerra-Sá R. de Oliveira DT, et al. Mol Biol Rep. 2020 Nov;47(11):9097-9122. doi: 10.1007/s11033-020-05916-3. Epub 2020 Oct 21. Mol Biol Rep. 2020. PMID: 33089404 Review. - Aberrant Methylation of LINE-1 Transposable Elements: A Search for Cancer Biomarkers.
Ponomaryova AA, Rykova EY, Gervas PA, Cherdyntseva NV, Mamedov IZ, Azhikina TL. Ponomaryova AA, et al. Cells. 2020 Sep 2;9(9):2017. doi: 10.3390/cells9092017. Cells. 2020. PMID: 32887319 Free PMC article. Review. - Evidence of epigenetic landscape shifts in mucopolysaccharidosis IIIB and IVA.
Vargas-López V, Prada LF, Alméciga-Díaz CJ. Vargas-López V, et al. Sci Rep. 2024 Feb 17;14(1):3961. doi: 10.1038/s41598-024-54626-4. Sci Rep. 2024. PMID: 38368436 Free PMC article. - Emergence of phenotypic plasticity through epigenetic mechanisms.
Romero-Mujalli D, Fuchs LIR, Haase M, Hildebrandt JP, Weissing FJ, Revilla TA. Romero-Mujalli D, et al. Evol Lett. 2024 Mar 27;8(4):561-574. doi: 10.1093/evlett/qrae012. eCollection 2024 Aug. Evol Lett. 2024. PMID: 39100234 Free PMC article.
References
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY 1999. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 23: 185–188 - PubMed
WWW RESOURCES
- http://www.illumina.com/products/methylation_450_beadchipkits.ilmn. Illumina methylation chip.
- http://www.sequenom.com/Sites/Genetic-Analysis/Applications/DNA-Methylation. Sequenom EpiTYPER.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources