Physical activity reduces hippocampal atrophy in elders at genetic risk for Alzheimer's disease - PubMed (original) (raw)
Physical activity reduces hippocampal atrophy in elders at genetic risk for Alzheimer's disease
J Carson Smith et al. Front Aging Neurosci. 2014.
Abstract
We examined the impact of physical activity (PA) on longitudinal change in hippocampal volume in cognitively intact older adults at varying genetic risk for the sporadic form of Alzheimer's disease (AD). Hippocampal volume was measured from structural magnetic resonance imaging (MRI) scans administered at baseline and at an 18-month follow-up in 97 healthy, cognitively intact older adults. Participants were classified as High or Low PA based on a self-report questionnaire of frequency and intensity of exercise. Risk status was defined by the presence or absence of the apolipoprotein E-epsilon 4 (APOE-ε4) allele. Four subgroups were studied: Low Risk/High PA (n = 24), Low Risk/Low PA (n = 34), High Risk/High PA (n = 22), and High Risk/Low PA (n = 17). Over the 18 month follow-up interval, hippocampal volume decreased by 3% in the High Risk/Low PA group, but remained stable in the three remaining groups. No main effects or interactions between genetic risk and PA were observed in control brain regions, including the caudate, amygdala, thalamus, pre-central gyrus, caudal middle frontal gyrus, cortical white matter (WM), and total gray matter (GM). These findings suggest that PA may help to preserve hippocampal volume in individuals at increased genetic risk for AD. The protective effects of PA on hippocampal atrophy were not observed in individuals at low risk for AD. These data suggest that individuals at genetic risk for AD should be targeted for increased levels of PA as a means of reducing atrophy in a brain region critical for the formation of episodic memories.
Keywords: Alzheimer's disease; association studies in genetics; cognitive aging; exercise; physical activity; volumetric MRI.
Figures
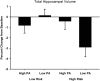
Figure 1
Percent change from baseline in total hippocampal volume for the four participant groups. Error bars represent s.e.m.
Similar articles
- Interactive effects of physical activity and APOE-ε4 on white matter tract diffusivity in healthy elders.
Smith JC, Lancaster MA, Nielson KA, Woodard JL, Seidenberg M, Durgerian S, Sakaie K, Rao SM. Smith JC, et al. Neuroimage. 2016 May 1;131:102-12. doi: 10.1016/j.neuroimage.2015.08.007. Epub 2015 Aug 8. Neuroimage. 2016. PMID: 26265157 Free PMC article. - Genetic risk for Alzheimer's disease alters the five-year trajectory of semantic memory activation in cognitively intact elders.
Rao SM, Bonner-Jackson A, Nielson KA, Seidenberg M, Smith JC, Woodard JL, Durgerian S. Rao SM, et al. Neuroimage. 2015 May 1;111:136-46. doi: 10.1016/j.neuroimage.2015.02.011. Epub 2015 Feb 14. Neuroimage. 2015. PMID: 25687593 Free PMC article. - Five-Year Longitudinal Brain Volume Change in Healthy Elders at Genetic Risk for Alzheimer's Disease.
Reiter K, Nielson KA, Durgerian S, Woodard JL, Smith JC, Seidenberg M, Kelly DA, Rao SM. Reiter K, et al. J Alzheimers Dis. 2017;55(4):1363-1377. doi: 10.3233/JAD-160504. J Alzheimers Dis. 2017. PMID: 27834774 Free PMC article. - Structural magnetic resonance imaging for the early diagnosis of dementia due to Alzheimer's disease in people with mild cognitive impairment.
Lombardi G, Crescioli G, Cavedo E, Lucenteforte E, Casazza G, Bellatorre AG, Lista C, Costantino G, Frisoni G, Virgili G, Filippini G. Lombardi G, et al. Cochrane Database Syst Rev. 2020 Mar 2;3(3):CD009628. doi: 10.1002/14651858.CD009628.pub2. Cochrane Database Syst Rev. 2020. PMID: 32119112 Free PMC article. - The APOE ε4 variant and hippocampal atrophy in Alzheimer's disease and Lewy body dementia: a systematic review of magnetic resonance imaging studies and therapeutic relevance.
Saeed U, Desmarais P, Masellis M. Saeed U, et al. Expert Rev Neurother. 2021 Aug;21(8):851-870. doi: 10.1080/14737175.2021.1956904. Epub 2021 Aug 13. Expert Rev Neurother. 2021. PMID: 34311631
Cited by
- APOE in the bullseye of neurodegenerative diseases: impact of the APOE genotype in Alzheimer's disease pathology and brain diseases.
Fernández-Calle R, Konings SC, Frontiñán-Rubio J, García-Revilla J, Camprubí-Ferrer L, Svensson M, Martinson I, Boza-Serrano A, Venero JL, Nielsen HM, Gouras GK, Deierborg T. Fernández-Calle R, et al. Mol Neurodegener. 2022 Sep 24;17(1):62. doi: 10.1186/s13024-022-00566-4. Mol Neurodegener. 2022. PMID: 36153580 Free PMC article. Review. - Association between APOE Genotype and Change in Physical Function in a Population-Based Swedish Cohort of Older Individuals Followed Over Four Years.
Skoog I, Hörder H, Frändin K, Johansson L, Östling S, Blennow K, Zetterberg H, Zettergren A. Skoog I, et al. Front Aging Neurosci. 2016 Oct 4;8:225. doi: 10.3389/fnagi.2016.00225. eCollection 2016. Front Aging Neurosci. 2016. PMID: 27757080 Free PMC article. - [Effect of physical exercise on Alzheimer's disease. A sistematic review].
Agüera Sánchez MÁ, Barbancho Ma MÁ, García-Casares N. Agüera Sánchez MÁ, et al. Aten Primaria. 2020 May;52(5):307-318. doi: 10.1016/j.aprim.2018.09.010. Epub 2019 May 29. Aten Primaria. 2020. PMID: 31153668 Free PMC article. Spanish. - Hippocampal and Cerebral Blood Flow after Exercise Cessation in Master Athletes.
Alfini AJ, Weiss LR, Leitner BP, Smith TJ, Hagberg JM, Smith JC. Alfini AJ, et al. Front Aging Neurosci. 2016 Aug 5;8:184. doi: 10.3389/fnagi.2016.00184. eCollection 2016. Front Aging Neurosci. 2016. PMID: 27547184 Free PMC article. - BDNF mediates improvements in executive function following a 1-year exercise intervention.
Leckie RL, Oberlin LE, Voss MW, Prakash RS, Szabo-Reed A, Chaddock-Heyman L, Phillips SM, Gothe NP, Mailey E, Vieira-Potter VJ, Martin SA, Pence BD, Lin M, Parasuraman R, Greenwood PM, Fryxell KJ, Woods JA, McAuley E, Kramer AF, Erickson KI. Leckie RL, et al. Front Hum Neurosci. 2014 Dec 11;8:985. doi: 10.3389/fnhum.2014.00985. eCollection 2014. Front Hum Neurosci. 2014. PMID: 25566019 Free PMC article.
References
- Dale A. M., Fischl B., Sereno M. I. (1999). Cortical surface-based analysis I: segmentation and surface reconstruction. Neuroimage 9, 179–194 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous