Antitumor activity of the combination of an HSP90 inhibitor and a PI3K/mTOR dual inhibitor against cholangiocarcinoma - PubMed (original) (raw)
. 2014 May 15;5(9):2372-89.
doi: 10.18632/oncotarget.1706.
Kun-Chun Chiang, Chi-Tung Cheng, Shih-Chiang Huang, Yeng-Yang Chen, Tsung-Wen Chen, Ta-Sen Yeh, Yi-Yin Jan, Hsi-Ming Wang, Jiang-Jie Weng, Peter Mu-Hsin Chang, Chun-Yu Liu, Chung-Pin Li, Yee Chao, Ming-Han Chen, Chi-Ying F Huang, Chun-Nan Yeh
Affiliations
- PMID: 24796583
- PMCID: PMC4058012
- DOI: 10.18632/oncotarget.1706
Antitumor activity of the combination of an HSP90 inhibitor and a PI3K/mTOR dual inhibitor against cholangiocarcinoma
Ming-Huang Chen et al. Oncotarget. 2014.
Abstract
The PI3K/Akt/mTOR pathway is overactivated and heat shock protein (HSP) 90 is overexpressed in common cancers. We hypothesized that targeting both pathways can kill intrahepatic cholangiocarcinoma (CCA) cells. HSP90 and PTEN protein expression was evaluated by immunohistochemical staining of samples from 78 patients with intrahepatic CCA. CCA cell lines and a thioacetamide (TAA)-induced CCA animal model were treated with NVP-AUY922 (an HSP90 inhibitor) and NVP-BEZ235 (a PI3K/mTOR inhibitor) alone or in combination. Both HSP90 overexpression and loss of PTEN were poor prognostic factors in patients with intrahepatic CCA. The combination of the HSP90 inhibitor NVP-AUY922 and the PI3K/mTOR inhibitor NVP-BEZ235 was synergistic in inducing cell death in CCA cells. A combination of NVP-AUY922 and NVP-BEZ235 caused tumor regression in CCA rat animal model. This combination not only inhibited the PI3K/Akt/mTOR pathway but also induced ROS, which may exacerbate the vicious cycle of ER stress. Our data suggest simultaneous targeting of the PI3K/mTOR and HSP pathways for CCA treatment.
Figures
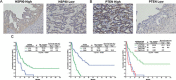
Figure 1. HSP90 and PTEN expression was correlated with survival in 78 patients with resectable MF-CCA
(A, B) Immunohistochemical staining of MF-CCA tumors with different intensity scores for HSP90 and PTEN expression; (C) The high-HSP90 group showed significantly worse overall survival (P < 0.001, left); The low-PTEN group showed significantly worse overall survival (P < 0.001, middle); The combined high-HSP90 and low-PTEN group showed the worst overall survival (P < 0.001, right).
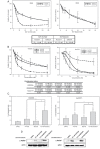
Figure 2. The combination of NVP-AUY922 and NVP-BEZ235 synergistically induced apoptosis in CCA cell lines
(A) CGCCA and HuCCT1 cells were incubated with various concentrations (0, 8, 16, 32, 64, or 128 nM) of either NVP-AUY922 or NVP-BEZ235 for 72 h; (B) CGCCA and HuCCT1 cells were incubated with either NVP-AUY922 or NVP-BEZ235 or both NVP-AUY922 and NVP-BEZ235 at various concentrations for 72 h. The combination index (CI) < 1, CI = 1, or CI > 1 indicate synergism, an additive effect, or antagonism, respectively; (C) CGCCA and HuCCT1 cells were treated with 0.5 uM of either NVP-AUY922 or NVP-BEZ235 or a combination of these 2 for 72 h. The number of apoptotic cells measured using the TACS Annexin V-FITC apoptosis detection kit is represented as a percentage of the total events. (D) The immunoblots are analyses of cleaved poly (ADP-ribose) polymerase (PARP). β-Actin was used as the loading control.
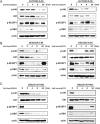
Figure 3. NVP-AUY922 and NVP-BEZ235 functioned together to block the PI3K/Akt/mTOR signaling pathway
(A) Western blot analysis revealed the molecular signature of PI3K/mTOR inhibition induced by NVP-AUY922 in the CCA and HuCCT1 cell lines. Cell lysates from the CGCCA and HuCCT1 cell lines were treated with 0.5 uM NVP-AUY922 at various time points (0, 24, 48, 72 h); (B) Western blot analysis reveals the molecular signature induced by NVP-BEZ235 in CCA and HuCCT1 cell lines. Cell lysates from CGCCA and HuCCT1 cells were treated with 0.5 uM NVP-BEZ235 for various time points (0, 24, 48, and 72 h); (C) Western blot analysis revealed the molecular signature induced by NVP-BEZ235 and NVP-AUY922 treatment in CCA and HuCCT1 cell lines. Cell lysates from CGCCA and HuCCT1 cells were treated with 0.5 uM NVP-AUY922 and NVP-BEZ235 at various time points (0, 24, 48, and 72 h). β-Actin was used as the loading control.
Figure 4. NVP-AUY922 induced ER stress and mitochondrial damage, which was fueled by oxidative stress when combined with NVP-BEZ235
(A) CCA cells were incubated with 0.5 uM NVP-AUY922 for 0, 2, 4, 8, 16, and 24 h. Whole cell lysates were subjected to western blot analysis for HSP70, Grp94, Grp78, p-eIF2α, CHOP, IRE1α, and phosphor-JNK. β-Actin was used as the loading control; (B) CCA cells were incubated with 0.5 uM NVP-AUY922 for 48 h. The red and green color ratio of JC-1 reflects the change in the mitochondrial membrane potential (ΔΨm); (C) Relative levels of reduced glutathione (GSH) in CGCCA cell line treated with 0.5 uM NVP-AUY922 and NVP-BEZ235 alone or combined for 0, 24, and 48 h. (D) Reactive oxidative species (ROS) levels induced by 0.5 uM NVP-AUY922 and NVP-BEZ235 alone or combined for 0, 18, and 24 h in CGCCA cell line. (E) The model shows that NVP-AUY922 induces ER stress, which leads to mitochondrial damage, and ultimately to apoptosis. When combined with NVP-BEZ235 treatment, this process is fueled by oxidative stress. NVP-BEZ235 and NVP-AUY922 cooperate to induce apoptosis by vertically affecting the PI3K/Akt/mTOR signaling pathway at multiple nodes.
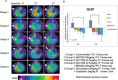
Figure 5. Detection of rat CCA by animal PET and changes in the tumor/liver SUV ratio
(A) Coronal views of fused CT and PET scans of control and experimental rats revealed the CCA-expressing areas of the liver in which the 18F-FDG uptake was higher than baseline at 2–5 wk after the experiment (i.e., wk 20, 22, and 25). (B) Change in the tumor-to-liver (T/L) ratio of SUV in the control and experiment groups at 2–5 wk after the experiment (i.e., wk 22 and 25).
Similar articles
- Increased activation of PI3K/AKT signaling pathway is associated with cholangiocarcinoma metastasis and PI3K/mTOR inhibition presents a possible therapeutic strategy.
Yothaisong S, Dokduang H, Techasen A, Namwat N, Yongvanit P, Bhudhisawasdi V, Puapairoj A, Riggins GJ, Loilome W. Yothaisong S, et al. Tumour Biol. 2013 Dec;34(6):3637-48. doi: 10.1007/s13277-013-0945-2. Epub 2013 Jul 6. Tumour Biol. 2013. PMID: 23832540 - Gene expression-based chemical genomics identifies heat-shock protein 90 inhibitors as potential therapeutic drugs in cholangiocarcinoma.
Chen MH, Lin KJ, Yang WL, Kao YW, Chen TW, Chao SC, Chang PM, Liu CY, Tzeng CH, Chao Y, Chen MH, Yeh CN, Huang CY. Chen MH, et al. Cancer. 2013 Jan 15;119(2):293-303. doi: 10.1002/cncr.27743. Epub 2012 Jul 18. Cancer. 2013. PMID: 22810956 - Combined targeting of AKT and mTOR using MK-2206 and RAD001 is synergistic in the treatment of cholangiocarcinoma.
Ewald F, Grabinski N, Grottke A, Windhorst S, Nörz D, Carstensen L, Staufer K, Hofmann BT, Diehl F, David K, Schumacher U, Nashan B, Jücker M. Ewald F, et al. Int J Cancer. 2013 Nov;133(9):2065-76. doi: 10.1002/ijc.28214. Epub 2013 May 29. Int J Cancer. 2013. PMID: 23588885 - Cholangiocarcinoma: molecular pathways and therapeutic opportunities.
Ilyas SI, Borad MJ, Patel T, Gores GJ. Ilyas SI, et al. Semin Liver Dis. 2014 Nov;34(4):456-64. doi: 10.1055/s-0034-1394144. Epub 2014 Nov 4. Semin Liver Dis. 2014. PMID: 25369307 Free PMC article. Review. - Role of dual PI3/Akt and mTOR inhibition in Waldenstrom's Macroglobulinemia.
Sacco A, Roccaro A, Ghobrial IM. Sacco A, et al. Oncotarget. 2010 Nov;1(7):578-582. doi: 10.18632/oncotarget.192. Oncotarget. 2010. PMID: 21317453 Free PMC article. Review.
Cited by
- CCL3 Promotes Proliferation of Colorectal Cancer Related with TRAF6/NF-_κ_B Molecular Pathway.
Ma X, Su J, Zhao S, He Y, Li S, Yang X, Zhai S, Rong S, Zhang X, Xu G, Xie X. Ma X, et al. Contrast Media Mol Imaging. 2022 Jul 12;2022:2387192. doi: 10.1155/2022/2387192. eCollection 2022. Contrast Media Mol Imaging. 2022. PMID: 35935327 Free PMC article. - WAVE3 Induces EMT and Promotes Migration and Invasion in Intrahepatic Cholangiocarcinoma.
Zhu Z, Chen W, Yin X, Lai J, Wang Q, Liang L, Wang W, Wang A, Zheng C. Zhu Z, et al. Dig Dis Sci. 2016 Jul;61(7):1950-60. doi: 10.1007/s10620-016-4102-9. Epub 2016 Mar 12. Dig Dis Sci. 2016. PMID: 26971088 - A novel HSP90 inhibitor with reduced hepatotoxicity synergizes with radiotherapy to induce apoptosis, abrogate clonogenic survival, and improve tumor control in models of colorectal cancer.
Kinzel L, Ernst A, Orth M, Albrecht V, Hennel R, Brix N, Frey B, Gaipl US, Zuchtriegel G, Reichel CA, Blutke A, Schilling D, Multhoff G, Li M, Niyazi M, Friedl AA, Winssinger N, Belka C, Lauber K. Kinzel L, et al. Oncotarget. 2016 Jul 12;7(28):43199-43219. doi: 10.18632/oncotarget.9774. Oncotarget. 2016. PMID: 27259245 Free PMC article. - Biomarkers in bile-complementing advanced endoscopic imaging in the diagnosis of indeterminate biliary strictures.
Lourdusamy V, Tharian B, Navaneethan U. Lourdusamy V, et al. World J Gastrointest Endosc. 2015 Apr 16;7(4):308-17. doi: 10.4253/wjge.v7.i4.308. World J Gastrointest Endosc. 2015. PMID: 25901209 Free PMC article. Review. - Immunosuppression in liver transplant oncology: position paper of the Italian Board of Experts in Liver Transplantation (I-BELT).
Cillo U, Carraro A, Avolio AW, Cescon M, Di Benedetto F, Giannelli V, Magistri P, Nicolini D, Vivarelli M, Lanari J; Italian Board of Experts in Liver Transplantation (I-BELT). Cillo U, et al. Updates Surg. 2024 Jun;76(3):725-741. doi: 10.1007/s13304-024-01845-z. Epub 2024 May 7. Updates Surg. 2024. PMID: 38713396 Review.
References
- Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. - PubMed
- Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. - PubMed
- Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. - PubMed
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous