Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota - PubMed (original) (raw)
Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota
Kei Arimatsu et al. Sci Rep. 2014.
Abstract
Periodontitis has been implicated as a risk factor for metabolic disorders such as type 2 diabetes, atherosclerotic vascular diseases, and non-alcoholic fatty liver disease. Although bacteremias from dental plaque and/or elevated circulating inflammatory cytokines emanating from the inflamed gingiva are suspected mechanisms linking periodontitis and these diseases, direct evidence is lacking. We hypothesize that disturbances of the gut microbiota by swallowed bacteria induce a metabolic endotoxemia leading metabolic disorders. To investigate this hypothesis, changes in the gut microbiota, insulin and glucose intolerance, and levels of tissue inflammation were analysed in mice after oral administration of Porphyromonas gingivalis, a representative periodontopathogens. Pyrosequencing revealed that the population belonging to Bacteroidales was significantly elevated in P. gingivalis-administered mice which coincided with increases in insulin resistance and systemic inflammation. In P. gingivalis-administered mice blood endotoxin levels tended to be higher, whereas gene expression of tight junction proteins in the ileum was significantly decreased. These results provide a new paradigm for the interrelationship between periodontitis and systemic diseases.
Figures
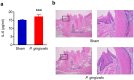
Figure 1. Systemic and local inflammation in _P. gingivalis_-administered and sham-administered mice (N = 8 in each group).
(a) Serum level of IL-6. All data are means ± SD. Significant differences were observed between the _P. gingivalis_-administered groups and the sham-administered group (***p < 0.001, Mann-Whitney U-test). (b) Histological findings of gingival tissues of _P. gingivalis_-administered and sham-administered mice. Sections of the periodontium around the disto-buccal root of the first molar were H-E stained. Right panels are magnified views of the boxed areas. No difference of gingival inflammation was observed between _P. gingivalis_-administered and sham-administered mice.
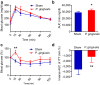
Figure 2. P. gingivalis induced insulin resistance.
(a) Intraperitoneal glucose tolerance test. Blood glucose levels were determined at the indicated times after intraperitoneal load of glucose (1 g/kg). (b) Area under blood concentration curve (AUC) of the Fig. 2a. (c) Intraperitoneal insulin tolerance test (ITT). Blood glucose levels were determined at the indicated times after intraperitoneal load of insulin (0.5 unit/kg). (N = 6 in each group). (d) Insulin sensitivity was assessed by inverse AUC of ITT. All data are means ± SD. (*p < 0.05; **p < 0.01, Mann-Whitney U-test).
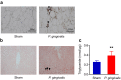
Figure 3. Relationship between P. gingivalis administration and inflammatory changes of epididymal adipose tissue and liver.
(a) Epididymal adipose tissues were fixed 10% formalin, sectioned, and stained with a rat anti-mouse F4/80 primary antibody (Abd Serotec). Arrow heads indicate F4/80 positive macrophages. (b) Oil red O staining of the liver tissue are shown. Increased number of lipid containing hepatocytes (arrow heads) are seen in _P. gingivalis_-administered mice. (c) P. gingivalis administration increased hepatic triglyceride content. (N = 8 in each group). All data are means ± SD. (**p < 0.01, Mann-Whitney U-test).
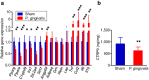
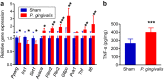
Figure 4. Effect of oral administration of P. gingivalis on the gene and protein expressions in epididymal adipose tissue.
(a) Comparison of relative gene expression levels in the adipose tissue between the _P. gingivalis_-administered and the sham-administered mice (N = 8 in each group). The relative quantity of experimental mRNA was normalized to the relative quantity of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. (b) CTRP9 contents in the epididymal adipose tissue were determined by ELISA of tissue lysates. All data are means ± SD. (*p < 0.05; **p < 0.01; ***p < 0.001, Mann-Whitney U-test).
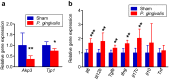
Figure 5. Effect of oral administration of P. gingivalis on the gene and protein expressions in liver.
(a) Comparison of relative gene expression levels in the liver tissue between the _P. gingivalis_-administered and the sham-administered mice (N = 8 in each group). The relative quantity of experimental mRNA was normalized to the relative quantity of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. (b) TNF-α levels in the liver tissue were determined by ELISA of tissue lysates. All data are means ± SD. (*p < 0.05; **p < 0.01; ***p < 0.001, Mann-Whitney U-test).
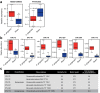
Figure 6. Comparison of the gut microbiota between _P. gingivalis_-administerd and sham-administered mice by 16S rRNA sequencing analysis.
Relative abundances of each bacterial group in Phylum (a) and OTU (b) level are indicated by boxplot. Each defined OTU obtained from 16S rRNA deep sequencing was compared to the genome database (GenomeDB) from NCBI using blastin (c). Close relative species and percent similarities are shown. Fold increases indicate the mean ratio of relative abundance of the OTU in the gut microbiota from the _P. gingivalis_-administered group to sham-administered group. (*p < 0.05, **p < 0.01, Mann-Whitney U-test).
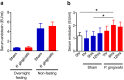
Figure 7. Effect of oral administration of P. gingivalis on the serum endotoxin levels.
(a) Serum endotoxin (LPS) concentration (EU/ml) were determined after 10 cycles of P. gingivalis administration or sham administration (N = 8 in each group). All data are means ± SD. (b) Serum endotoxin (LPS) concentration (EU/ml) were determined at the indicated times after single administration of P. gingivalis (N = 6 in each group). All data are means ± SD. (*p < 0.05, one-way ANOVA).
Figure 8. Comparison of relative gene expression levels in the small intestine (a) and colon tissue (b) between the sham-administered group and the _P. gingivalis_-administered group (N = 8 in each group).
The relative quantity of experimental mRNA was normalized to the relative quantity of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. All data are means ± SD. (*p < 0.05; **p < 0.01, ***p < 0.001, Mann-Whitney U-test).
Similar articles
- Oral Administration of P. gingivalis Induces Dysbiosis of Gut Microbiota and Impaired Barrier Function Leading to Dissemination of Enterobacteria to the Liver.
Nakajima M, Arimatsu K, Kato T, Matsuda Y, Minagawa T, Takahashi N, Ohno H, Yamazaki K. Nakajima M, et al. PLoS One. 2015 Jul 28;10(7):e0134234. doi: 10.1371/journal.pone.0134234. eCollection 2015. PLoS One. 2015. PMID: 26218067 Free PMC article. - An orally administered oral pathobiont and commensal have comparable and innocuous systemic effects in germ-free mice.
Sato K, Yokoji M, Yamada M, Nakajima T, Yamazaki K. Sato K, et al. J Periodontal Res. 2018 Dec;53(6):950-960. doi: 10.1111/jre.12593. Epub 2018 Jul 26. J Periodontal Res. 2018. PMID: 30047130 - Ligature-induced periodontitis in mice induces elevated levels of circulating interleukin-6 but shows only weak effects on adipose and liver tissues.
Matsuda Y, Kato T, Takahashi N, Nakajima M, Arimatsu K, Minagawa T, Sato K, Ohno H, Yamazaki K. Matsuda Y, et al. J Periodontal Res. 2016 Oct;51(5):639-46. doi: 10.1111/jre.12344. Epub 2015 Dec 15. J Periodontal Res. 2016. PMID: 26667667 - Oral microbiota-induced periodontitis: a new risk factor of metabolic diseases.
Minty M, Canceil T, Serino M, Burcelin R, Tercé F, Blasco-Baque V. Minty M, et al. Rev Endocr Metab Disord. 2019 Dec;20(4):449-459. doi: 10.1007/s11154-019-09526-8. Rev Endocr Metab Disord. 2019. PMID: 31741266 Review. - Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury.
Frazier TH, DiBaise JK, McClain CJ. Frazier TH, et al. JPEN J Parenter Enteral Nutr. 2011 Sep;35(5 Suppl):14S-20S. doi: 10.1177/0148607111413772. Epub 2011 Aug 1. JPEN J Parenter Enteral Nutr. 2011. PMID: 21807932 Review.
Cited by
- Thermosensitive Hydrogels for Periodontal Regeneration: A Systematic Review of the Evidence.
Amiri MA, Amiri D, Hamedani S. Amiri MA, et al. Clin Exp Dent Res. 2024 Dec;10(6):e70029. doi: 10.1002/cre2.70029. Clin Exp Dent Res. 2024. PMID: 39539029 Free PMC article. Review. - Evaluation of Periodontitis and Fusobacterium nucleatum Among Colorectal Cancer Patients: An Observational Cross-Sectional Study.
Antonacci A, Bizzoca C, Barile G, Andriola V, Vincenti L, Bartolomeo N, Abbinante A, Orrù G, Corsalini M. Antonacci A, et al. Healthcare (Basel). 2024 Nov 4;12(21):2189. doi: 10.3390/healthcare12212189. Healthcare (Basel). 2024. PMID: 39517401 Free PMC article. - Oral Pathobiont-Derived Outer Membrane Vesicles in the Oral-Gut Axis.
Catalan EA, Seguel-Fuentes E, Fuentes B, Aranguiz-Varela F, Castillo-Godoy DP, Rivera-Asin E, Bocaz E, Fuentes JA, Bravo D, Schinnerling K, Melo-Gonzalez F. Catalan EA, et al. Int J Mol Sci. 2024 Oct 17;25(20):11141. doi: 10.3390/ijms252011141. Int J Mol Sci. 2024. PMID: 39456922 Free PMC article. Review. - Experimental peri-implantitis induces neuroinflammation: An exploratory study in rats.
Cafferata EA, Ramanauskaite A, Cuypers A, Obreja K, Dohle E, Ghanaati S, Schwarz F. Cafferata EA, et al. BMC Oral Health. 2024 Oct 18;24(1):1238. doi: 10.1186/s12903-024-04995-z. BMC Oral Health. 2024. PMID: 39425138 Free PMC article. - Balancing the Oral-Gut-Brain Axis with Diet.
Kerstens R, Ng YZ, Pettersson S, Jayaraman A. Kerstens R, et al. Nutrients. 2024 Sep 22;16(18):3206. doi: 10.3390/nu16183206. Nutrients. 2024. PMID: 39339804 Free PMC article. Review.
References
- Petersen P. E. & Ogawa H. Strengthening the prevention of periodontal disease: the WHO approach. J Periodontol. 76, 2187–2193 (2005). - PubMed
- Bahekar A. A., Singh S., Saha S., Molnar J. & Arora R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: a meta-analysis. Am Heart J. 154, 830–837 (2007). - PubMed
- Chávarry N. G., Vettore M. V., Sansone C. & Sheiham A. The relationship between diabetes mellitus and destructive periodontal disease: a meta-analysis. Oral Health Prev Dent. 7, 107–127 (2009). - PubMed
- Salvi G. E., Carollo-Bittel B. & Lang N. P. Effects of diabetes mellitus on periodontal and peri-implant conditions: update on associations and risks. J Clin Periodontol. 35, 398–409 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical