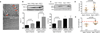Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle - PubMed (original) (raw)
. 2014 May 9;344(6184):649-52.
doi: 10.1126/science.1251152. Epub 2014 May 5.
Young C Jang, Juhyun Oh, Danika Khong, Elizabeth Y Wu, Rohan Manohar, Christine Miller, Samuel G Regalado, Francesco S Loffredo, James R Pancoast, Michael F Hirshman, Jessica Lebowitz, Jennifer L Shadrach, Massimiliano Cerletti, Mi-Jeong Kim, Thomas Serwold, Laurie J Goodyear, Bernard Rosner, Richard T Lee, Amy J Wagers
Affiliations
- PMID: 24797481
- PMCID: PMC4104429
- DOI: 10.1126/science.1251152
Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle
Manisha Sinha et al. Science. 2014.
Abstract
Parabiosis experiments indicate that impaired regeneration in aged mice is reversible by exposure to a young circulation, suggesting that young blood contains humoral "rejuvenating" factors that can restore regenerative function. Here, we demonstrate that the circulating protein growth differentiation factor 11 (GDF11) is a rejuvenating factor for skeletal muscle. Supplementation of systemic GDF11 levels, which normally decline with age, by heterochronic parabiosis or systemic delivery of recombinant protein, reversed functional impairments and restored genomic integrity in aged muscle stem cells (satellite cells). Increased GDF11 levels in aged mice also improved muscle structural and functional features and increased strength and endurance exercise capacity. These data indicate that GDF11 systemically regulates muscle aging and may be therapeutically useful for reversing age-related skeletal muscle and stem cell dysfunction.
Figures
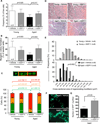
Figure 1. Rejuvenation of muscle stem cells by heterochronic parabiosis
(A). Frequency of clone-sorted satellite cells from isochronic (Iso) or heterochronic (Het) mice forming colonies after 5 days in culture. All colonies showed characteristic morphology of muscle lineage cells. (B) DNA damage in freshly sorted satellite cells assessed by single cell gel electrophoresis under alkaline conditions. Damage was quantified using a visual scoring metric (25) (key at top) and represented by color-coding: no damage (green), moderate damage (orange), maximal damage (red). (C) Representative images (confocal z-stacks) of freshly sorted satellite cells stained with DAPI (blue) and anti-pH2AX (green); data are quantified in (D). All graphs represent mean ± SD, with _p_-values, calculated by Mann-Whitney analysis. “n=” indicates number of mice used for each analysis. Scale bar = 10µm.
Figure 2. Rejuvenation of muscle stem cells by rGDF11 supplementation
(A, B). Frequency (A) and myogenic colony formation (B) of satellite cells from vehicle- or rGDF11-treated mice. (C). Quantification of DNA damage assays using freshly sorted satellite cells from vehicle- or rGDF11-treated mice, scored as in Fig. 1B. (D, E). H&E staining (D) and frequency distribution of myofiber size (E) in regenerating TA muscles 7 days after cryoinjury in vehicle- or rGDF11-treated young and aged mice. Scale bar = 100µm. (F). Representative images of transverse cryosections of TA muscles 2 weeks after transplantation. (G) Quantification of transplant data as maximal number of GFP+ myofibers found in each engrafted muscle. Graphs represent mean ± SD (in A–C, G). _p_-values were calculated by Mann-Whitney analysis (A–C), Wilcoxon Exact analysis (E), or Student’s _t_-test (G). “n=” indicates number of mice used for each analysis.
Figure 3. Improved muscle physiology and physical function after rGDF11 supplementation
(A). Electron micrographs of transverse sections of TA muscle from vehicle- or rGDF11-treated aged mice (representative of n=4 per group). Arrows indicate swollen mitochondria. (B, C). Western blot of PGC-1α (B) and LC3 forms I and II (C) in TA muscle extracts from cardiotoxin-injured or uninjured vehicle- or rGDF11-treated aged mice. Three animals are shown for each experimental group. Densitometric quantification of Western data are provided below each blot, normalized to GAPDH (B) or Actin (C). (D, E). Scatter plots of exercise endurance (D, maximum treadmill runtime in a 90 minute window) or forelimb grip-strength (E) of vehicle- or rGDF11-treated aged mice. Grip-strength is plotted as maximum force (Newton, N) exerted in triplicate trials. Red line represents the maximum grip-strength of 33-39 week-old young male mice. Data are presented for individual mice (black symbols) overlaid with mean ± SD (orange lines). _p_-values were calculated by Mann-Whitney analysis. “n=” indicates number of mice used for each analysis.
Comment in
- Brain ageing: Blood-derived rejuvenation.
Yates D. Yates D. Nat Rev Neurosci. 2014 Jun;15(6):352-3. doi: 10.1038/nrn3763. Nat Rev Neurosci. 2014. PMID: 24840795 No abstract available.
Similar articles
- Brain ageing: Blood-derived rejuvenation.
Yates D. Yates D. Nat Rev Neurosci. 2014 Jun;15(6):352-3. doi: 10.1038/nrn3763. Nat Rev Neurosci. 2014. PMID: 24840795 No abstract available. - Regenerative medicine. 'Rejuvenating' protein doubted.
Kaiser J. Kaiser J. Science. 2015 May 22;348(6237):849. doi: 10.1126/science.348.6237.849. Science. 2015. PMID: 25999487 No abstract available. - Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors.
Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Katsimpardi L, et al. Science. 2014 May 9;344(6184):630-4. doi: 10.1126/science.1251141. Epub 2014 May 5. Science. 2014. PMID: 24797482 Free PMC article. - Growth differentiation factor 11: a "rejuvenation factor" involved in regulation of age-related diseases?
Ma Y, Liu Y, Han F, Qiu H, Shi J, Huang N, Hou N, Sun X. Ma Y, et al. Aging (Albany NY). 2021 Apr 22;13(8):12258-12272. doi: 10.18632/aging.202881. Epub 2021 Apr 22. Aging (Albany NY). 2021. PMID: 33886503 Free PMC article. Review. - The role of GDF11 in aging and skeletal muscle, cardiac and bone homeostasis.
Egerman MA, Glass DJ. Egerman MA, et al. Crit Rev Biochem Mol Biol. 2019 Apr;54(2):174-183. doi: 10.1080/10409238.2019.1610722. Crit Rev Biochem Mol Biol. 2019. PMID: 31144559 Review.
Cited by
- Interorgan Communication Pathways in Physiology: Focus on Drosophila.
Droujinine IA, Perrimon N. Droujinine IA, et al. Annu Rev Genet. 2016 Nov 23;50:539-570. doi: 10.1146/annurev-genet-121415-122024. Epub 2016 Oct 10. Annu Rev Genet. 2016. PMID: 27732790 Free PMC article. Review. - Contribution of muscle satellite cells to sarcopenia.
Huo F, Liu Q, Liu H. Huo F, et al. Front Physiol. 2022 Aug 12;13:892749. doi: 10.3389/fphys.2022.892749. eCollection 2022. Front Physiol. 2022. PMID: 36035464 Free PMC article. Review. - Interactions between muscle stem cells, mesenchymal-derived cells and immune cells in muscle homeostasis, regeneration and disease.
Farup J, Madaro L, Puri PL, Mikkelsen UR. Farup J, et al. Cell Death Dis. 2015 Jul 23;6(7):e1830. doi: 10.1038/cddis.2015.198. Cell Death Dis. 2015. PMID: 26203859 Free PMC article. Review. - Novel insights into the pleiotropic health effects of growth differentiation factor 11 gained from genome-wide association studies in population biobanks.
Strosahl J, Ye K, Pazdro R. Strosahl J, et al. BMC Genomics. 2024 Sep 6;25(1):837. doi: 10.1186/s12864-024-10710-7. BMC Genomics. 2024. PMID: 39237910 Free PMC article. - Control of stress signaling in stem cells: crossroads of stem cells and cancer.
Cho SJ, Koo J, Chun KH, Cha HJ. Cho SJ, et al. Tumour Biol. 2016 Oct;37(10):12983-12990. doi: 10.1007/s13277-016-5249-x. Epub 2016 Jul 27. Tumour Biol. 2016. PMID: 27460084 Review.
References
- Jang YC, Sinha M, Cerletti M, Dall'osso C, Wagers AJ. Skeletal Muscle Stem Cells: Effects of Aging and Metabolism on Muscle Regenerative Function. Cold Spring Harb Symp Quant Biol. 2011 Sep 29; - PubMed
- Sherwood RI, et al. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004 Nov 12;119:543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR042238/AR/NIAMS NIH HHS/United States
- 5U01 HL100402/HL/NHLBI NIH HHS/United States
- 1R01 AG033053/AG/NIA NIH HHS/United States
- 1R01 AG040019/AG/NIA NIH HHS/United States
- DP2 OD004345/OD/NIH HHS/United States
- 1DP2 OD004345/OD/NIH HHS/United States
- R01 AR42238/AR/NIAMS NIH HHS/United States
- T32 DE007057/DE/NIDCR NIH HHS/United States
- R01 AG033053/AG/NIA NIH HHS/United States
- R01 AG032977/AG/NIA NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 AG040019/AG/NIA NIH HHS/United States
- U01 HL100402/HL/NHLBI NIH HHS/United States
- P30 DK036836/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases