Lysosomal adaptation: how the lysosome responds to external cues - PubMed (original) (raw)
Lysosomal adaptation: how the lysosome responds to external cues
Carmine Settembre et al. Cold Spring Harb Perspect Biol. 2014.
Abstract
Recent evidence indicates that the importance of the lysosome in cell metabolism and organism physiology goes far beyond the simple disposal of cellular garbage. This dynamic organelle is situated at the crossroad of the most important cellular pathways and is involved in sensing, signaling, and transcriptional mechanisms that respond to environmental cues, such as nutrients. Two main mediators of these lysosomal adaptation mechanisms are the mTORC1 kinase complex and the transcription factor EB (TFEB). These two factors are linked in a lysosome-to-nucleus signaling pathway that provides the lysosome with the ability to adapt to extracellular cues and control its own biogenesis. Modulation of lysosomal function by acting on TFEB has a profound impact on cellular clearance and energy metabolism and is a promising therapeutic target for a large variety of disease conditions.
Copyright © 2014 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
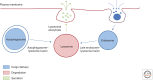
Figure 1.
The lysosome and cellular clearance. The figure depicts the three major aspects of lysosome-mediated cellular clearance: cargo targeting to lysosomes, substrate degradation, and secretion. Lysosomes receive extracellular material via endocytosis and intracellular material via autophagy. In these processes, lysosomes fuse with late endosomes and with autophagosomes, respectively. Subsequently, substrates are degraded by lysosomal hydrolases and the resulting breakdown products are used to generate new cellular components and energy in response to the nutritional needs of the cell. Lysosomes also undergo Ca2+-regulated exocytosis to secrete their content into the extracellular space and to repair damaged plasma membranes.
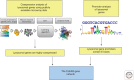
Figure 2.
Identification of the CLEAR gene network. A systems biology approach was used to test the hypothesis that lysosomal genes are coregulated. The analysis of the expression behavior of genes encoding lysosomal proteins was analyzed using publicly available microarray data and revealed that they are coexpressed. Pattern discovery analysis revealed the presence of a palindromic 10-base site in the promoters of known lysosomal genes. This sequence was previously identified as a specific version of a known target site for basic helix–loop–helix (bHLH) transcription factors, also known as an E-box. Thus, these two independent approaches—namely, coexpression and promoter analyses—identified a new gene network that was named CLEAR (coordinated lysosomal expression and regulation). envir., environment.
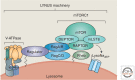
Figure 3.
The LYNUS machinery. A complex machinery, made of several interacting protein complexes was identified on the lysosomal surface. This machinery, herein named lysosomal nutrient sensing (LYNUS), includes regulatory proteins associated with mTOR, such as RAPTOR (regulatory-associated protein of mTOR), mLST8 (mammalian lethal with SEC13 protein), and DEPTOR (DEP domain-containing mTOR-interacting protein), and Rag GTPases (RagA or RagB and RagC or RagD), which activate mTORC1 on the lysosomal surface. A complex known as Ragulator mediates the activation and docking of Rag GTPases to the lysosomal membrane. The small GTPase Ras homolog enriched in brain (Rheb) is also involved in the growth factor-mediated activation of mTORC1. The V-ATPase complex functions in amino acid sensing and mediates amino acid-sensitive interactions between Rag GTPases and Ragulator, which is the initial step in lysosomal signaling. The endolysosomal ATP-sensitive Na+-permeable channel (lysoNaATP), which comprises the subunits two pore calcium channel 1 (TPC1) and TPC2, is located on the lysosomal membrane, and it has recently been shown to interact with mTORC1 and to participate in nutrient sensing. (Figure is based on data from Settembre et al. 2013b.)
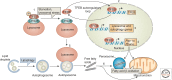
Figure 4.
Model of TFEB regulation and function during starvation. This model illustrates how TFEB mediates the starvation response by regulating lipid catabolism. In the presence of sufficient nutrients, TFEB interacts with the LYNUS machinery, which senses lysosomal nutrient levels via the vacuolar ATPase (V-ATPase) complex, and is phosphorylated by mammalian target of rapamycin complex 1 (mTORC1) on the lysosomal surface (1). This keeps TFEB inactive by cytosolic sequestration. During starvation, mTORC1 is released from the LYNUS machinery and becomes inactive. Thus, TFEB can no longer be phosphorylated by mTORC1 and translocates to the nucleus, where it induces its own transcription (2). Therefore, starvation regulates TFEB activity through a dual mechanism that involves a posttranslational modification (that is, phosphorylation) and a transcriptional autoregulatory loop. Once in the nucleus, TFEB regulates the expression of genes involved in the lysosomal–autophagy pathway (3), as well as of _Ppar_-α (peroxisome proliferator-activated receptor-α) and _Pgc_-1α (PPAR-γ coactivator 1α) and their target genes (4). In this way, TFEB controls the starvation response by activating both lipophagy (5) and fatty acid β-oxidation (6). (Figure is based on data from Settembre et al. 2013a,.)
Figure 5.
TFEB as a tool to promote cellular clearance in human disease. Human diseases for which viral-mediated TFEB-overexpression-induced cellular clearance and ameliorated disease phenotype in mouse models.
Similar articles
- A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB.
Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard MC, Facchinetti V, Sabatini DM, Ballabio A. Settembre C, et al. EMBO J. 2012 Mar 7;31(5):1095-108. doi: 10.1038/emboj.2012.32. Epub 2012 Feb 17. EMBO J. 2012. PMID: 22343943 Free PMC article. - The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis.
Roczniak-Ferguson A, Petit CS, Froehlich F, Qian S, Ky J, Angarola B, Walther TC, Ferguson SM. Roczniak-Ferguson A, et al. Sci Signal. 2012 Jun 12;5(228):ra42. doi: 10.1126/scisignal.2002790. Sci Signal. 2012. PMID: 22692423 Free PMC article. - Giant Cellular Vacuoles Induced by Rare Earth Oxide Nanoparticles are Abnormally Enlarged Endo/Lysosomes and Promote mTOR-Dependent TFEB Nucleus Translocation.
Lin J, Shi SS, Zhang JQ, Zhang YJ, Zhang L, Liu Y, Jin PP, Wei PF, Shi RH, Zhou W, Wen LP. Lin J, et al. Small. 2016 Nov;12(41):5759-5768. doi: 10.1002/smll.201601903. Epub 2016 Sep 4. Small. 2016. PMID: 27593892 - TFEB at a glance.
Napolitano G, Ballabio A. Napolitano G, et al. J Cell Sci. 2016 Jul 1;129(13):2475-81. doi: 10.1242/jcs.146365. Epub 2016 Jun 1. J Cell Sci. 2016. PMID: 27252382 Free PMC article. Review. - Transcription factor EB: from master coordinator of lysosomal pathways to candidate therapeutic target in degenerative storage diseases.
Sardiello M. Sardiello M. Ann N Y Acad Sci. 2016 May;1371(1):3-14. doi: 10.1111/nyas.13131. Ann N Y Acad Sci. 2016. PMID: 27299292 Free PMC article. Review.
Cited by
- Pompe disease: from pathophysiology to therapy and back again.
Lim JA, Li L, Raben N. Lim JA, et al. Front Aging Neurosci. 2014 Jul 23;6:177. doi: 10.3389/fnagi.2014.00177. eCollection 2014. Front Aging Neurosci. 2014. PMID: 25183957 Free PMC article. Review. - Unconventional secretion of FABP4 by endosomes and secretory lysosomes.
Villeneuve J, Bassaganyas L, Lepreux S, Chiritoiu M, Costet P, Ripoche J, Malhotra V, Schekman R. Villeneuve J, et al. J Cell Biol. 2018 Feb 5;217(2):649-665. doi: 10.1083/jcb.201705047. Epub 2017 Dec 6. J Cell Biol. 2018. PMID: 29212659 Free PMC article. - Senescent Cells: Emerging Targets for Human Aging and Age-Related Diseases.
Song S, Lam EW, Tchkonia T, Kirkland JL, Sun Y. Song S, et al. Trends Biochem Sci. 2020 Jul;45(7):578-592. doi: 10.1016/j.tibs.2020.03.008. Epub 2020 Apr 6. Trends Biochem Sci. 2020. PMID: 32531228 Free PMC article. Review. - Up-regulation of lysosomal TRPML1 channels is essential for lysosomal adaptation to nutrient starvation.
Wang W, Gao Q, Yang M, Zhang X, Yu L, Lawas M, Li X, Bryant-Genevier M, Southall NT, Marugan J, Ferrer M, Xu H. Wang W, et al. Proc Natl Acad Sci U S A. 2015 Mar 17;112(11):E1373-81. doi: 10.1073/pnas.1419669112. Epub 2015 Mar 2. Proc Natl Acad Sci U S A. 2015. PMID: 25733853 Free PMC article. - Micro-Transcriptome Analysis Reveals Immune-Related MicroRNA Regulatory Networks of Paralichthys olivaceus Induced by Vibrio anguillarum Infection.
Ning X, Sun L. Ning X, et al. Int J Mol Sci. 2020 Jun 15;21(12):4252. doi: 10.3390/ijms21124252. Int J Mol Sci. 2020. PMID: 32549342 Free PMC article.
References
- Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R 2004. Mutations in the glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. N Engl J Med 351: 1972–1977 - PubMed
- Ballabio A, Gieselmann V 2009. Lysosomal disorders: From storage to cellular damage. Biochim Biophys Acta 1793: 684–696 - PubMed
- Bar-Peled L, Sabatini DM 2012. SnapShot: mTORC1 signaling at the lysosomal surface. Cell 151: 1390–1390 e1391 - PubMed
- Bargal R, Avidan N, Ben-Asher E, Olender Z, Zeigler M, Frumkin A, Raas-Rothschild A, Glusman G, Lancet D, Bach G 2000. Identification of the gene causing mucolipidosis type IV. Nat Genet 26: 118–123 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS078072/NS/NINDS NIH HHS/United States
- R01 NS078072-01A1/NS/NINDS NIH HHS/United States
- TGM11CB6/TI_/Telethon/Italy
- TCP12008/TI_/Telethon/Italy
- 250154/ERC_/European Research Council/International
LinkOut - more resources
Full Text Sources
Other Literature Sources