Inhibition of the JAK2/STAT3 pathway reduces gastric cancer growth in vitro and in vivo - PubMed (original) (raw)
Inhibition of the JAK2/STAT3 pathway reduces gastric cancer growth in vitro and in vivo
Louise M Judd et al. PLoS One. 2014.
Abstract
Signal Transducer and Activator of Transcription-3 (STAT3) is constitutively activated in many cancers where it promotes growth, inflammation, angiogenesis and inhibits apoptosis. We have shown that STAT3 is constitutively activated in human gastric cancer, and that chronic IL-11-driven STAT3 transcriptional activity induces gastric tumourigenesis in the gp130(757FF) mouse model of gastric cancer development. Here we show that treatment of human AGS gastric cancer cells with the Janus Kinase (JAK) inhibitor WP1066 dose-, and time-dependently inhibits STAT3 phosphorylation, in conjunction with reduced JAK2 phosphorylation, reduced proliferation and increased apoptosis. In addition, application of intraperitoneal WP1066 for 2 weeks, reduced gastric tumour volume by 50% in the gp130(757FF) mouse coincident with reduced JAK2 and STAT3 activation compared with vehicle-treated, littermate controls. Gastric tumours from WP1066- treated mice had reduced polymorphonuclear inflammation, coincident with inhibition of numerous proinflammatory cytokines including IL-11, IL-6 and IL-1β, as well as the growth factors Reg1 and amphiregulin. These results show that WP1066 can block proliferation, reduce inflammation and induce apoptosis in gastric tumour cells by inhibiting STAT3 phosphorylation, and that many cytokines and growth factors that promote gastric tumour growth are regulated by STAT3-dependent mechanisms. WP1066 may form the basis for future therapeutics against gastric cancer.
Conflict of interest statement
Competing Interests: Waldemar Priebe hold patents and has a financial interest in the development of compound WP1066 as follows; W.Priebe, N.Donato, M.Talpaz, S, Szymanski, I. Fokt, A. Levitzki Compounds for the treatment of cell proliferative diseases. Unites States Patent WO/2005/058829 12/01/2004. Note also that the authors have provided an amended statement of Competing Interests regarding a patent on WP1066 held by W. Priebe. The authors also declare that “this does not alter our adherence to PLOS ONE policies on sharing data and materials”.
Figures
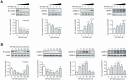
Figure 1. WP1066 modulates STAT3 and MAPK signalling endpoints in AGS cells.
A. Dose-response: AGS cells were treated with WP1066 at 0, 1, 2 and 5 µM for 60 min and extracts immunoblotted with antibodies specific for (i) pJAK2 and JAK2; (ii) pSTAT3 and STAT3; (iii) pERK1/2 and ERK1/2; (iv) pSHP2 and SHP2. Data was also expressed as a ratio of the phosphorylated to total protein product in each case. The outcomes of 3 experiments are shown as mean OD ratios±standard error (SE). Statistical significance for each treatment compared to 0 µM WP1066 is shown if p<0.05. B. Time-course: AGS cells were treated as in Fig. 1A but with 5 µM WP1066 for 0, 15, 30, 60, and 180 min. Data was treated as for Fig. 1A.
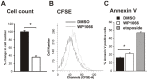
Figure 2. WP1066 inhibits proliferation and stimulates apoptosis of AGC cells.
Proliferation: A. AGS cells were treated with WP1066 at 5 µM for 18 hr, stained with trypan blue and viable cells counted. Data are expressed as the percentage of viable cells compared to vehicle alone. N = 3, mean±SE. B. CFSE tracking was used to measure changes in AGS cell proliferation after treatment with WP 1066. The raw fluorescence data collected is shown compared to DMSO controls for a representative experiment. Apoptosis: C. AGS cells treated for 24 hr with DMSO (method control), WP1066 (5 µM) or etoposide (200 µM; positive control) and adherent cells were stained with Annexin V then counted manually. Data are expressed as fold change compared to DMSO vehicle from a representative experiment. *p = 0.047.
Figure 3. Treatment of gp130757FF mice with WP1066 reduces tumour size.
gp130757FF mice (8 weeks old) were treated i.p with 10 mg/kg of WP1066 or DMSO twice with a gap of one day between doses, followed by doses of 20 mg/kg every 2 days for 2 weeks. Antral tumours in 8 week untreated mice (A) and 10 week DMSO-treated vehicle controls (B) were no different in mean area, however WP1066 treatment resulted in ∼50% smaller tumours (C). This was confirmed by quantitative morphometry (D) where antral tumours were significantly smaller in WP1066 mice compared to 10 w.o. DMSO controls and 8 w.o. untreated mice (n = 7–10; p<0.05). The effect of WP1066 on cell proliferation was assessed by staining sections with an antibody for Ki-67. There was a significant decrease in the number of Ki-67 positive cells per gland in the WP1066 treated mice (F) compared to controls (E) and quantified graphically (G). The number of apoptotic cells quantified after caspase 3 staining of antral sections of a DMSO control (H) and WP1066 (I) mouse, and was quantified by counting stained cells/mm2 of gastric mucosa (J).
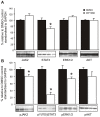
Figure 4. WP1066 inhibits STAT3 signalling.
gp130757FF mice (8 weeks old) were treated as in Fig. 3 and antral extracts quantified by Western blotting for total JAK2, STAT3, ERK1/2 and AKT (A) and pJAK2, pSTAT3, pERK1/2 and pAKT (B). Membranes were concurrently hybridised with a GAPDH antibody as a loading control. The intensity of the signal was quantified by densitometry and expressed as a percentage of the signal relative to the controls standardised to the intensity of the GAPDH signal. n = 6–10; *significantly (p<0.05).
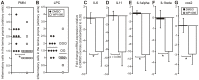
Figure 5. WP1066 treatment of gp130757FF mice inhibits the pro-inflammatory response in the antral mucosa.
gp130757FF mice (8 weeks old) treated as for Fig. 3, had antral stomach tissue taken for histology and pro-inflammatory gene expression by Q-PCR after mRNA extraction. Treatment of gp130757FF mice with WP1066 caused a significant reduction (p<0.05) in polymorphonuclear infiltrate (A) in the antral stomach, but lymphoplaysmocytic infiltrate was unchanged (B). Gene expression relative to the housekeeper GAPDH for IL-6 (C), IL-11 (D), IL-1 α (E), IL-1β (F) and COX-2 (G), was quantified by the ΔΔCT method. All n = 8; *p<0.05 compared to DMSO controls.
Figure 6. WP1066 treatment of gp130757FF mice inhibits growth factor expression in the antral mucosa.
gp130757FF mice (8 weeks old) were treated as for Fig. 5. mRNA for Reg1, amphiregulin and HB-EGF were analysed by Q-PCR analysis and quantified by the ΔΔCT method. EGFR ligands were differentially regulated so that amphiregulin mRNA was significantly decreased in the WP1066 treated mice compared to controls (p<0.05), while HB-EGF was unchanged. The non-EGFR ligand RegI showed a strong trend towards decreased expression (p = 0.069). All n = 8; *statistically different (p≤0.05).
Similar articles
- WP1066 disrupts Janus kinase-2 and induces caspase-dependent apoptosis in acute myelogenous leukemia cells.
Ferrajoli A, Faderl S, Van Q, Koch P, Harris D, Liu Z, Hazan-Halevy I, Wang Y, Kantarjian HM, Priebe W, Estrov Z. Ferrajoli A, et al. Cancer Res. 2007 Dec 1;67(23):11291-9. doi: 10.1158/0008-5472.CAN-07-0593. Cancer Res. 2007. PMID: 18056455 - On-target JAK2/STAT3 inhibition slows disease progression in orthotopic xenografts of human glioblastoma brain tumor stem cells.
Stechishin OD, Luchman HA, Ruan Y, Blough MD, Nguyen SA, Kelly JJ, Cairncross JG, Weiss S. Stechishin OD, et al. Neuro Oncol. 2013 Feb;15(2):198-207. doi: 10.1093/neuonc/nos302. Epub 2012 Dec 21. Neuro Oncol. 2013. PMID: 23262510 Free PMC article. - JAK2 inhibitor blocks the inflammation and growth of esophageal squamous cell carcinoma in vitro through the JAK/STAT3 pathway.
Fang J, Chu L, Li C, Chen Y, Hu F, Zhang X, Zhao H, Liu Z, Xu Q. Fang J, et al. Oncol Rep. 2015 Jan;33(1):494-502. doi: 10.3892/or.2014.3609. Epub 2014 Nov 14. Oncol Rep. 2015. PMID: 25405520 - Cytokine signalling via gp130 in gastric cancer.
Howlett M, Menheniott TR, Judd LM, Giraud AS. Howlett M, et al. Biochim Biophys Acta. 2009 Nov;1793(11):1623-33. doi: 10.1016/j.bbamcr.2009.07.009. Epub 2009 Aug 7. Biochim Biophys Acta. 2009. PMID: 19665497 Review. - Interleukin-6 serves as a critical factor in various cancer progression and therapy.
Mohamed AH, Ahmed AT, Al Abdulmonem W, Bokov DO, Shafie A, Al-Hetty HRAK, Hsu CY, Alissa M, Nazir S, Jamali MC, Mudhafar M. Mohamed AH, et al. Med Oncol. 2024 Jun 20;41(7):182. doi: 10.1007/s12032-024-02422-5. Med Oncol. 2024. PMID: 38900329 Review.
Cited by
- SENP1-mediated deSUMOylation of JAK2 regulates its kinase activity and platinum drug resistance.
Li J, Wu R, Yung MMH, Sun J, Li Z, Yang H, Zhang Y, Liu SS, Cheung ANY, Ngan HYS, Braisted JC, Zheng W, Wei H, Gao Y, Nemes P, Pei H, Chan DW, Li Y, Zhu W. Li J, et al. Cell Death Dis. 2021 Apr 1;12(4):341. doi: 10.1038/s41419-021-03635-6. Cell Death Dis. 2021. PMID: 33795649 Free PMC article. - Molecular analysis of V617F mutation in Janus kinase 2 gene of breast cancer patients.
Karim S, Malik IR, Nazeer Q, Zaheer A, Farooq M, Mahmood N, Malik A, Asif M, Mehmood A, Khan AR, Jabbar A, Arshad M, Yousafi Q, Hussain A, Mirza Z, Iqbal MA, Rasool M. Karim S, et al. Saudi J Biol Sci. 2019 Sep;26(6):1123-1128. doi: 10.1016/j.sjbs.2019.08.002. Epub 2019 Aug 2. Saudi J Biol Sci. 2019. PMID: 31516339 Free PMC article. - Glycosylation of Methylflavonoids in the Cultures of Entomopathogenic Filamentous Fungi as a Tool for Obtaining New Biologically Active Compounds.
Krawczyk-Łebek A, Dymarska M, Janeczko T, Kostrzewa-Susłow E. Krawczyk-Łebek A, et al. Int J Mol Sci. 2022 May 16;23(10):5558. doi: 10.3390/ijms23105558. Int J Mol Sci. 2022. PMID: 35628367 Free PMC article. - Delayed Administration of WP1066, an STAT3 Inhibitor, Ameliorates Radiation-Induced Lung Injury in Mice.
Yu J, Yuan X, Liu Y, Zhang K, Wang J, Zhang H, Liu F. Yu J, et al. Lung. 2016 Feb;194(1):67-74. doi: 10.1007/s00408-015-9821-8. Epub 2015 Nov 13. Lung. 2016. PMID: 26563331 - Single agent BMS-911543 Jak2 inhibitor has distinct inhibitory effects on STAT5 signaling in genetically engineered mice with pancreatic cancer.
Mace TA, Shakya R, Elnaggar O, Wilson K, Komar HM, Yang J, Pitarresi JR, Young GS, Ostrowski MC, Ludwig T, Bekaii-Saab T, Bloomston M, Lesinski GB. Mace TA, et al. Oncotarget. 2015 Dec 29;6(42):44509-22. doi: 10.18632/oncotarget.6332. Oncotarget. 2015. PMID: 26575024 Free PMC article.
References
- Burke WM, Jin X, Lin HJ, Huang M, Liu R, et al. (2001) Inhibition of constitutively active Stat3 suppresses growth of human ovarian and breast cancer cells. Oncogene 20: 7925–7934. - PubMed
- Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, et al. (2003) Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res 63: 1270–1279. - PubMed
- Mora LB, Buettner R, Seigne J, Diaz J, Ahmad N, et al. (2002) Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res 62: 6659–6666. - PubMed
- Mora LB, Buettner R, Ahmad N, Bassel Y, Jove R, et al. (2001) Prostate adenocarcinoma: cellular and molecular abnormalities. Cancer Control 8: 551–556. - PubMed
- Jackson CB, Judd LM, Menheniott TR, Kronborg I, Dow C, et al. (2007) Augmented gp130-mediated cytokine signalling accompanies human gastric cancer progression. J Pathol 213: 140–151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous