Phase I study of olaratumab in Japanese patients with advanced solid tumors - PubMed (original) (raw)
Clinical Trial
Phase I study of olaratumab in Japanese patients with advanced solid tumors
Toshihiko Doi et al. Cancer Sci. 2014 Jul.
Abstract
Olaratumab (IMC-3G3) is a fully human IgG1 monoclonal antibody that selectively binds the external domain of human platelet-derived growth factor receptor-α with high affinity and blocks ligand binding. This was a single-center, dose-escalation, phase I trial of olaratumab in Japanese patients with advanced/refractory solid malignancies. Three to six patients were enrolled into each of three cohorts: Patients received i.v. olaratumab: 10 mg/kg on days 1 and 8 every 3 weeks (cohort 1); 20 mg/kg every 2 weeks (cohort 2); and 15 mg/kg on days 1 and 8 every 3 weeks (cohort 3). Doses were escalated from cohort 1 through cohort 3. The primary objective was to establish the safety and pharmacokinetic profile of olaratumab. Sixteen patients were treated across three cohorts. There were no dose-limiting toxicities, so the maximum tolerated dose was not reached. The most common olaratumab-related treatment-emergent adverse events (TEAEs) were proteinuria (25.0%) and elevated aspartate transaminase (12.5%). One patient (cohort 2) had two olaratumab-related Grade 3 TEAEs (increased aspartate aminotransferase and tumor hemorrhage); otherwise, olaratumab-related TEAEs were Grade 1/2. Seven patients (43.8%) had a best response of stable disease. Based on the pharmacokinetic concentration profile of olaratumab, the trough concentrations following single and multiple doses at 15 mg/kg on days 1 and 8 every 3 weeks (cohort 3) and multiple doses at 20 mg/kg every 2 weeks (cohort 2) were above the 155 μg/mL target. Thus, these two doses could represent an acceptable schedule for future trials in Japanese patients. Olaratumab had an acceptable safety profile and was well tolerated.
Keywords: IMC-3G3; monoclonal antibody; olaratumab; phase 1; platelet-derived growth factor receptor.
© 2014 The Authors. Cancer Science published by Wiley Publishing Asia Pty Ltd on behalf of Japanese Cancer Association.
Figures
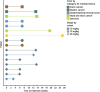
Figure 1
Time on treatment. The duration of treatment for each patient is shown.
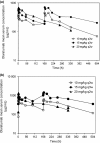
Figure 2
Arithmetic mean olaratumab serum concentration versus time profiles following the first dose (a) and multiple (b) doses of olaratumab. Semi-log scales are shown in each plot. h, hour; q2w, every 2 weeks; q3w, every 3 weeks.
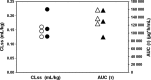
Figure 3
Comparison of PK parameters clearance and exposure between non-Asian and Asian patients following multiple infusions of olaratumab. Shown are CLss (circles) and AUCτ (triangles) for the 20 mg/kg-every-2-weeks groups. White circles (○) = CLss, non-Asian patients; black circles (•) = CLss, Asian patients; white triangles (▵) = AUCτ, non-Asian patients; black triangles (▴) = AUCτ Asian patients. AUCτ, area under the concentration versus time curve during one dosing interval; CLss, total body clearance of drug calculated after intravenous administration at steady state.
Similar articles
- A phase I study of olaratumab, an anti-platelet-derived growth factor receptor alpha (PDGFRα) monoclonal antibody, in patients with advanced solid tumors.
Chiorean EG, Sweeney C, Youssoufian H, Qin A, Dontabhaktuni A, Loizos N, Nippgen J, Amato R. Chiorean EG, et al. Cancer Chemother Pharmacol. 2014 Mar;73(3):595-604. doi: 10.1007/s00280-014-2389-9. Epub 2014 Jan 23. Cancer Chemother Pharmacol. 2014. PMID: 24452395 Clinical Trial. - Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial.
Heery CR, O'Sullivan-Coyne G, Madan RA, Cordes L, Rajan A, Rauckhorst M, Lamping E, Oyelakin I, Marté JL, Lepone LM, Donahue RN, Grenga I, Cuillerot JM, Neuteboom B, Heydebreck AV, Chin K, Schlom J, Gulley JL. Heery CR, et al. Lancet Oncol. 2017 May;18(5):587-598. doi: 10.1016/S1470-2045(17)30239-5. Epub 2017 Mar 31. Lancet Oncol. 2017. PMID: 28373007 Free PMC article. Clinical Trial. - A phase II study of a human anti-PDGFRα monoclonal antibody (olaratumab, IMC-3G3) in previously treated patients with metastatic gastrointestinal stromal tumors.
Wagner AJ, Kindler H, Gelderblom H, Schöffski P, Bauer S, Hohenberger P, Kopp HG, Lopez-Martin JA, Peeters M, Reichardt P, Qin A, Nippgen J, Ilaria RL, Rutkowski P. Wagner AJ, et al. Ann Oncol. 2017 Mar 1;28(3):541-546. doi: 10.1093/annonc/mdw659. Ann Oncol. 2017. PMID: 28426120 Free PMC article. Clinical Trial. - Olaratumab: A Novel Platelet-Derived Growth Factor Receptor α-Inhibitor for Advanced Soft Tissue Sarcoma.
Andrick BJ, Gandhi A. Andrick BJ, et al. Ann Pharmacother. 2017 Dec;51(12):1090-1098. doi: 10.1177/1060028017723935. Epub 2017 Aug 4. Ann Pharmacother. 2017. PMID: 28778132 Review. - Olaratumab for soft tissue sarcoma.
Teyssonneau D, Italiano A. Teyssonneau D, et al. Expert Opin Biol Ther. 2017 Aug;17(8):1019-1025. doi: 10.1080/14712598.2017.1339031. Epub 2017 Jul 10. Expert Opin Biol Ther. 2017. PMID: 28691538 Review.
Cited by
- PDGF/PDGFR effects in osteosarcoma and the "add-on" strategy.
Xu J, Xie L, Guo W. Xu J, et al. Clin Sarcoma Res. 2018 Aug 2;8:15. doi: 10.1186/s13569-018-0102-1. eCollection 2018. Clin Sarcoma Res. 2018. PMID: 30083310 Free PMC article. Review. - Incidence and Management of Olaratumab Infusion-Related Reactions.
Van Tine BA, Govindarajan R, Attia S, Somaiah N, Barker SS, Shahir A, Barrett E, Lee P, Wacheck V, Ramage SC, Tap WD. Van Tine BA, et al. J Oncol Pract. 2019 Nov;15(11):e925-e933. doi: 10.1200/JOP.18.00761. Epub 2019 Jul 3. J Oncol Pract. 2019. PMID: 31268811 Free PMC article. - The Combination of Olaratumab with Doxorubicin and Cisplatinum Regresses a Chemotherapy-Resistant Osteosarcoma in a Patient-Derived Orthotopic Xenograft Mouse Model.
Higuchi T, Sugisawa N, Miyake K, Oshiro H, Yamamoto N, Hayashi K, Kimura H, Miwa S, Igarashi K, Bouvet M, Singh SR, Tsuchiya H, Hoffman RM. Higuchi T, et al. Transl Oncol. 2019 Sep;12(9):1257-1263. doi: 10.1016/j.tranon.2019.06.002. Epub 2019 Jul 9. Transl Oncol. 2019. PMID: 31299622 Free PMC article. - Spotlight on olaratumab in the treatment of soft-tissue sarcoma: design, development, and place in therapy.
Davis EJ, Chugh R. Davis EJ, et al. Drug Des Devel Ther. 2017 Dec 13;11:3579-3587. doi: 10.2147/DDDT.S121298. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 29263653 Free PMC article. Review. - Drug Monographs: Olaratumab and Rucaparib.
Solimando DA Jr, Waddell JA. Solimando DA Jr, et al. Hosp Pharm. 2017 Apr;52(4):258-263. doi: 10.1310/hpj5203-258. Hosp Pharm. 2017. PMID: 28515503 Free PMC article.
References
- Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–316. - PubMed
- Adams SF, Hickson JA, Hutto JY, Montag AG, Lengyel E, Yamada SD. PDGFR-alpha as a potential therapeutic target in uterine sarcomas. Gynecol Oncol. 2007;104:524–8. - PubMed
- Fleming TP, Saxena A, Clark WC, et al. Amplification and/or overexpression of platelet-derived growth factor receptors and epidermal growth factor receptor in human glial tumors. Cancer Res. 1992;52:4550–3. - PubMed
- Matei D, Emerson RE, Lai YC, et al. Autocrine activation of PDGFRalpha promotes the progression of ovarian cancer. Oncogene. 2006;25:2060–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources