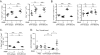Targeting antigens through blood dendritic cell antigen 2 on plasmacytoid dendritic cells promotes immunologic tolerance - PubMed (original) (raw)
Targeting antigens through blood dendritic cell antigen 2 on plasmacytoid dendritic cells promotes immunologic tolerance
Craig P Chappell et al. J Immunol. 2014.
Abstract
The C-type lectin receptor blood dendritic cell Ag 2 (BDCA2) is expressed exclusively on human plasmacytoid DCs (pDCs) and plays a role in Ag capture, internalization, and presentation to T cells. We used transgenic mice that express human BDCA2 and anti-BDCA2 mAbs to deliver Ags directly to BDCA2 on pDCs in vivo. Targeting Ag to pDCs in this manner resulted in significant suppression of Ag-specific CD4(+) T cell and Ab responses upon secondary exposure to Ag in the presence of adjuvant. Suppression of Ab responses required both a decrease in effector CD4(+) T cells and preservation of Foxp3(+) regulatory T cells (Tregs). Reduction in Treg numbers following Ag delivery to BDCA2 restored both CD4(+) T cell activation and Ab responses, demonstrating that Tregs were required for the observed tolerance. Our results demonstrate that Ag delivery to pDCs through BDCA2 is an effective method to induce immunological tolerance, which may be useful for treating autoimmune diseases or to inhibit unwanted Ab responses.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures
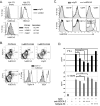
Figure 1. Characterization of anti-human BDCA2 mAbs and human BDCA2 transgenic mice
A, upper panel, NIH-3T3 cells transfected with BDCA2 or an empty vector control were stained with 10 μg/ml anti-BDCA2 mAb, UW80.1 or mouse IgG1 isotype control (G28-1). Lower panel, NIH-3T3-BDCA2 transfectants were incubated with either UW80.1 anti-BDCA2, mIgG1 isotype control or left untreated. Following washes to remove unbound mAbs, cells were stained with 5 μl PE-conjugated anti-BDCA2, (AC144). B, Analysis of pDC-specific expression of BDCA2. Splenocytes from control B6 or B6.BDCA2 transgenic mice were stained with fluorochrome-conjugated mAbs against CD11c, PDCA-1, Siglec-H, B220, and BDCA2 (UW80.1) or mIgG1 isotype control. Upper panel shows BDCA2 expression on PDCA-1+ pDCs from B6 and B6.BDCA2 Tg mice. Lower panel displays Siglec-H and B220 expression on BDCA2-and CD11c-gated cells from B6.BDCA2 Tg mice. Numbers in plots denote frequency among total scatter-gated splenocytes. Data shown are representative of >10 animals analyzed. C, Splenocytes from B6.BDCA2 Tg mice were stained with mAbs against CD19, CD4, CD8, NK1.1, CD11c, Siglec-H, PDCA-1, and BDCA2 or mIgG1 isotype control. Data shown are representative of 3 animals analyzed. D, Magnetically enriched pDCs from B6.BDCA2 Tg mice were stimulated in vitro with 10 μg/ml CpG-A with or without 2 or 10 μg/ml BDCA2 mAb (AC144) or mIgG1 isotype control (G28-1). 18 h later supernatants were collected and assayed for IFN-α and IL-12p40 by ELISA. Experiment shown is representative of 4 independent experiments.
Figure 2. BDCA2 can serve as a target for Ag delivery in B6.BDCA2 mice
A, Groups of B6.BDCA2 mice were injected i.v. with 2 μg AlexaFluor647-conjugated anti-BDCA2 (UW80.1) or mIgG1 isotype control and sacrificed one hour following injection. Splenocytes from injected mice were stained with mAbs specific for PDCA-1, Siglec-H, and CD11c, or IgD and CD19. Flow plots (left) depict gating for Siglec-H+PDCA-1+ pDCs (upper), CD11c+ myeloid DCs (middle), and CD19+IgD+ B cells (bottom). Numbers within flow plots denote frequency among total splenocytes. Histograms (right) show AlexaFluor647 fluorescence among the gated populations from anti-BDCA2-647-injected or mIgG1-647-injected mice. Numbers within histograms denote frequency of AlexaFluor647-positive cells among the gated population from mice that received anti-BDCA2-647. A representative experiment of five is shown using 2-3 mice/group. B, Groups of B6.BDCA2 mice were immunized i.v. with 10 μg OVA-conjugated anti-BDCA2, mIgG1 isotype control or anti-DCIR2 with or without 50 μg CpG-A. Graph depicts mean ± SEM of anti-OVA IgG in the serum 10 d post-immunization. A representative experiment of three is shown using 3-4 mice/group.
Figure 3. Targeting Ag to BDCA2+ pDCs leads to a reduction of Ag-specific T cells
A, Schematic for immunization and subsequent analysis. B, B6.BDCA2 recipients of CFSE-labeled OT-II cells were injected i.v. with PBS or 1 μg OVA-isotype, OVA-BDCA2 or OVA-DEC205 and sacrificed on d 4 following injection. Flow plots (top) depict gating of CD4+Ly5.1+ cells. Histograms (bottom) depict CFSE fluorescence among gated CD4+Ly5.1+ population in each group. C-D, Cumulative data from B showing the percentage of divided cells (C) and cell proliferation index (D) defined as total CFSE fluorescence (MFI) of PBS group divided by total CFSE fluorescence (MFI) for each group. Data in B-D are from 2 independent experiments using 2-4 mice per group. E, Cohorts of B6.BDCA2 recipients were immunized as in (A) and the frequency of CD4+Ly5.1+ and CD4+Ly5.1− cells were determined at d 4. Numbers within flow plots denote frequency among total splenocytes. F, Scatter plots depict the total number of CD4+Ly5.1+ splenocytes from mice immunized as in (A) 4, 7 and 14 d post-immunization. Data in E-F are from three independent experiments using 3 mice/group. G, The number of endogenous CD4+ T cells per spleen 7 d p.i. from mice immunized as in (A) are plotted. H, The percentage of apoptotic cells within gated CD4+Ly5.1+ splenocytes at d 4 and d 7 was determined by AnnexinV staining in B6.BDCA2 recipients immunized as in (A). Data in C, F and G are pooled data from three independent experiments where each dot represents an individual animal with the mean indicated for each group (horizontal bars) ± SEM. Cell numbers in F and G were calculated based on the total cell number per spleen. * p<0.05, ** p<0.01, *** p<0.001, as determined by two-tailed, unpaired Student’s t-test or one-way ANOVA with Tukey post-test.
Figure 4. Targeting Ag to BDCA2+ pDCs leads to increased frequencies of Ag-specific Treg cells
A-C, Cohorts of B6.BDCA2 recipients were immunized as in (Fig. 3A) and sacrificed 7 d post-immunization. A, The frequency of Foxp3+ cells within CD4+Ly5.1+ and CD4+Ly5.1− splenocytes is shown. B and C, Combined data from three independent experiments showing the frequency and number of Foxp3+CD4+Ly5.1+ (B) and Foxp3+CD4+Ly5.1− splenocytes (C). D, Histograms depict CFSE dilution among gated Foxp3+CD4+Ly5.1+ splenocytes 4 d following immunization as indicated. E, Representative histograms show the expression of Helios and CD25 among gated Foxp3+CD4+Ly5.1+ cells 7 d p.i.. F, Representative histograms show CD44 expression among gated Foxp3+CD4+Ly5.1+ cells 7 d p.i. For B, C and F, each dot represents an individual animal with the mean indicated for each group (horizontal bars) ± SEM. Cell numbers in B and C were calculated based on the total cell number per spleen. * p<0.05, ** p<0.01, *** p<0.001, as determined by two-tailed, unpaired Student’s t-test or one-way ANOVA with Tukey post-test.
Figure 5. Ag delivery to BDCA2+ pDCs alters secondary T-cell responses and leads to increased frequencies of Foxp3+ T cells
A, Schematic for immunization and subsequent analysis for panels B-E. B, Flow plots depict gating strategy for analysis of CD44 and CD62L expression among transferred CD4+Ly5.1+ Foxp3− and Foxp3+ splenocytes. Numbers in quadrants represent percentages within the gated population. C, Data showing the frequencies and numbers of CD4+Ly5.1+ cells. D, Scatter plots depict the frequency and number of Foxp3+ CD4+ Ly5.1+ cells. E, Scatter plots show the ratio of Foxp3+ CD4+ Ly5.1+ to Foxp3− CD4+ Ly5.1+ splenocytes as defined in B. Data shown in C, D and E are pooled data from three independent experiments. Each dot represents an individual animal with the mean indicated for each group (horizontal bars) ± SEM. Cell numbers in C and D were calculated based on the total cell number per spleen. F. Cohorts of B6.BDCA2 recipients of OT-II T cells were injected with PBS, 1 μg OVA-isotype 1 (mouse IgG1), OVA-BDCA2, OVA-isotype 2 (rat IgG2a), OVA-SiglecH or OVA-DEC205; mice were otherwise treated as in A and analyzed as in B. Scatter plot depicts the frequency of Foxp3+CD4+Ly5.1+ splenocytes within each group. Data are pooled from two independent experiments. Each dot represents an individual animal with the mean indicated for each group (horizontal bars). * p<0.05, ** p<0.01, *** p<0.001, as determined by two-tailed, unpaired Student’s t-test or one-way ANOVA with Tukey post-test.
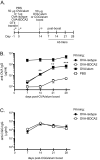
Figure 6. OVA-BDCA2 immunization suppresses Ag-specific Ab responses
A, Schematic for immunization and subsequent analysis. B, Cohorts of B6.BDCA2 recipients of OT-II T cells were primed with 1 μg OVA-BDCA2 or OVA-isotype, or given PBS or 50μg OVA plus alum one day following adoptive transfer. 14 d later mice were boosted with 50 μg OVA plus alum and then bled weekly for 4 wks to collect serum. Line graph depicts quantities of OVA-specific serum IgG as determined by ELISA. Data shown are a representative experiment of 3 independent experiments using 3-4 mice/group. C, B6.BDCA2 recipients of OT-II T cells were primed with 1 μg OVA-BDCA2 or OVA-isotype and challenged 14 d later with either 50 μg OVA or CGG plus alum and bled weekly for 4 wks to collect serum. Line graph shows quantities of OVA-specific serum IgG as determined by ELISA. A representative experiment of 3 independent experiments using 3-4 mice/group is shown.
Figure 7. Depletion of Tregs by anti-CD25 Ab restores Ag-specific Ab responses following Ag re-challenge
A, Schematic for immunization, injections and subsequent analysis. B, Flow plots depict frequencies of CD4+Ly5.1+ and CD4+Ly5.1− cells (among total splenocytes) and Foxp3+ and Foxp3− cells (among gated CD4+Ly5.1+ cells) in mice given anti-CD25 or isotype control mAbs 8 d previously. C, Scatter plot showing the frequency of Foxp3+ cells among gated CD4+Ly5.1+ splenocytes for the indicated groups. D, Scatter plot showing the frequency of CD4+Ly5.1+ cells among total splenocytes for the indicated groups. E, Flow plots depicting CD44 and CD62L expression among gated Foxp3− CD4+ Ly5.1+ splenocytes. F, Scatter plot depicts the combined frequency of activated CD44hiCD62Lhi/lo cells gated in (E). Data in B-F are from three independent experiments using 2-3 mice/group. For C, D and F, each dot represents an individual animal with the mean indicated for each group (horizontal bars) ± SEM. G, Line graph depicts quantities of OVA-specific serum IgG as determined by ELISA for the indicated groups. Data shown are the combined results from 3 independent experiments using 3-5 mice/group. * p<0.05, ** p<0.01, *** p<0.001, as determined by one-way ANOVA with Tukey post-test (B-F) or one-way ANOVA with repeated measures (G).
Figure 8. TLR7 agonist treatment at the time of Ag delivery prevents Teff cell deletion and restores Ag-specific Ab responses following Ag re-challenge
Cohorts of B6.BDCA2 mice were immunized as in Fig. 3A with or without 50 μg R848. A, Scatter plots showing the frequency (left) and number (right) of total transferred Ly5.1+ CD4+ splenocytes for the indicated groups 7 d p.i. B, Scatter plots showing the frequency (left) and number (right) of Foxp3+ cells among CD4+ Ly5.1+ splenocytes quantified in A. C, Scatter plot depicts the ratio of the frequencies of Foxp3+ CD4+ Ly5.1+ to Foxp3− CD4+ Ly5.1+ for the indicated groups at d 7 p.i. Data shown in _A-C a_re from 2 independent experiments using 3-4 mice/group. Each dot represents an individual animal with the mean indicated for each group (horizontal bars) ± SEM. Cell numbers were calculated based on the total cell number per spleen. E. Line graph shows the quantity of OVA-specific serum IgG for the indicated groups as determined by ELISA. One representative experiment of three independent experiments using 3-5 mice/group is shown. Each dot represents an individual animal with the mean indicated for each group (horizontal bars) ± SEM. * p<0.05, ** p<0.01, *** p<0.001, as determined by one-way ANOVA with Tukey post-test.
Similar articles
- Anti-BDCA2 monoclonal antibody inhibits plasmacytoid dendritic cell activation through Fc-dependent and Fc-independent mechanisms.
Pellerin A, Otero K, Czerkowicz JM, Kerns HM, Shapiro RI, Ranger AM, Otipoby KL, Taylor FR, Cameron TO, Viney JL, Rabah D. Pellerin A, et al. EMBO Mol Med. 2015 Apr;7(4):464-76. doi: 10.15252/emmm.201404719. EMBO Mol Med. 2015. PMID: 25762615 Free PMC article. - BDCA2/Fc epsilon RI gamma complex signals through a novel BCR-like pathway in human plasmacytoid dendritic cells.
Cao W, Zhang L, Rosen DB, Bover L, Watanabe G, Bao M, Lanier LL, Liu YJ. Cao W, et al. PLoS Biol. 2007 Sep 11;5(10):e248. doi: 10.1371/journal.pbio.0050248. PLoS Biol. 2007. PMID: 17850179 Free PMC article. - Human BDCA2+CD123+CD56+ dendritic cells (DCs) related to blastic plasmacytoid dendritic cell neoplasm represent a unique myeloid DC subset.
Yu H, Zhang P, Yin X, Yin Z, Shi Q, Cui Y, Liu G, Wang S, Piccaluga PP, Jiang T, Zhang L. Yu H, et al. Protein Cell. 2015 Apr;6(4):297-306. doi: 10.1007/s13238-015-0140-x. Epub 2015 Mar 18. Protein Cell. 2015. PMID: 25779340 Free PMC article. - Dendritic cells expand antigen-specific Foxp3+ CD25+ CD4+ regulatory T cells including suppressors of alloreactivity.
Yamazaki S, Inaba K, Tarbell KV, Steinman RM. Yamazaki S, et al. Immunol Rev. 2006 Aug;212:314-29. doi: 10.1111/j.0105-2896.2006.00422.x. Immunol Rev. 2006. PMID: 16903923 Review. - Targeted antigen delivery to DEC-205⁺ dendritic cells for tolerogenic vaccination.
Petzold C, Schallenberg S, Stern JN, Kretschmer K. Petzold C, et al. Rev Diabet Stud. 2012 Winter;9(4):305-18. doi: 10.1900/RDS.2012.9.305. Epub 2012 Dec 28. Rev Diabet Stud. 2012. PMID: 23804268 Free PMC article. Review.
Cited by
- Modulation of Human Dendritic Cell Functions by Phosphodiesterase-4 Inhibitors: Potential Relevance for the Treatment of Respiratory Diseases.
Nguyen HO, Tiberio L, Facchinetti F, Ripari G, Violi V, Villetti G, Salvi V, Bosisio D. Nguyen HO, et al. Pharmaceutics. 2023 Aug 31;15(9):2254. doi: 10.3390/pharmaceutics15092254. Pharmaceutics. 2023. PMID: 37765223 Free PMC article. Review. - Unboxing dendritic cells: Tales of multi-faceted biology and function.
Giza HM, Bozzacco L. Giza HM, et al. Immunology. 2021 Nov;164(3):433-449. doi: 10.1111/imm.13394. Epub 2021 Aug 8. Immunology. 2021. PMID: 34309853 Free PMC article. Review. - Emerging glyco-based strategies to steer immune responses.
Anderluh M, Berti F, Bzducha-Wróbel A, Chiodo F, Colombo C, Compostella F, Durlik K, Ferhati X, Holmdahl R, Jovanovic D, Kaca W, Lay L, Marinovic-Cincovic M, Marradi M, Ozil M, Polito L, Reina-Martin JJ, Reis CA, Sackstein R, Silipo A, Švajger U, Vaněk O, Yamamoto F, Richichi B, van Vliet SJ. Anderluh M, et al. FEBS J. 2021 Aug;288(16):4746-4772. doi: 10.1111/febs.15830. Epub 2021 May 15. FEBS J. 2021. PMID: 33752265 Free PMC article. Review. - Plasmacytoid dendritic cell biology and its role in immune-mediated diseases.
Ye Y, Gaugler B, Mohty M, Malard F. Ye Y, et al. Clin Transl Immunology. 2020 May 26;9(5):e1139. doi: 10.1002/cti2.1139. eCollection 2020 May. Clin Transl Immunology. 2020. PMID: 32489664 Free PMC article. Review. - Targeting Antigens to CD180 but Not CD40 Programs Immature and Mature B Cell Subsets to Become Efficient APCs.
Roe K, Shu GL, Draves KE, Giordano D, Pepper M, Clark EA. Roe K, et al. J Immunol. 2019 Oct 1;203(7):1715-1729. doi: 10.4049/jimmunol.1900549. Epub 2019 Sep 4. J Immunol. 2019. PMID: 31484732 Free PMC article.
References
- Caminschi I, Lahoud MH, Shortman K. Enhancing immune responses by targeting antigen to DC. Eur J Immunol. 2009;39:931–938. - PubMed
- Kreutz M, Tacken PJ, Figdor CG. Targeting dendritic cells--why bother? Blood. 2013;121:2836–2844. - PubMed
- Geijtenbeek TB, van Vliet SJ, Engering A, t Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu Rev Immunol. 2004;22:33–54. - PubMed
- Garcia-Vallejo JJ, van Kooyk Y. The physiological role of DC-SIGN: A tale of mice and men. Trends Immunol. 2009;34:482–486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI44257/AI/NIAID NIH HHS/United States
- R01 AI052203/AI/NIAID NIH HHS/United States
- R37 AI044257/AI/NIAID NIH HHS/United States
- R01 AI044257/AI/NIAID NIH HHS/United States
- AI52203/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials