3,4-Methylenedioxypyrovalerone (MDPV) and metabolites quantification in human and rat plasma by liquid chromatography-high resolution mass spectrometry - PubMed (original) (raw)
3,4-Methylenedioxypyrovalerone (MDPV) and metabolites quantification in human and rat plasma by liquid chromatography-high resolution mass spectrometry
Sebastien Anizan et al. Anal Chim Acta. 2014.
Abstract
Synthetic cathinones are recreational drugs that mimic the effects of illicit stimulants like cocaine, amphetamine or Ecstasy. Among the available synthetic cathinones in the United States, 3,4-methylenedioxypyrovalerone (MDPV) is commonly abused and associated with dangerous side effects. MDPV is a dopamine transporter blocker 10-fold more potent than cocaine as a locomotor stimulant in rats. Previous in vitro and in vivo studies examining MDPV metabolism reported 3,4-dihydroxypyrovalerone (3,4-catechol-PV) and 4-hydroxy-3-methoxypyrovalerone (4-OH-3-MeO-PV) as the two primary metabolites. We developed and validated a liquid chromatography-high resolution mass spectrometry method to quantify MDPV and its primary metabolites in 100 μL human and rat plasma. Plasma hydrolysis was followed by protein precipitation before analysis. Limits of detection were 0.1 μg L(-1), with linear ranges from 0.25 to 1000 μg L(-1). Process efficiency, matrix effect, total imprecision (%CV) and accuracy (%target) were 36-93%, from -8 to 12%, 2.1 to 7.3% and 86 to 109%, respectively. MDPV and metabolites were stable at room temperature for 24 h, 4 °C for 72 h and after 3 freeze-thaw cycles with less than 10% variability. Human-rat plasma cross validation demonstrated that rat plasma could be accurately quantified against a human plasma calibration curve. As proof of this method, rat plasma specimens were analyzed after intraperitoneal and subcutaneous dosing with MDPV (0.5 mg kg(-1)). MDPV, 3,4-catechol-PV and 4-OH-3-MeO-PV concentrations ranged from not detected to 107.5 μg L(-1) prior to and up to 8h after dosing. This method provides a simultaneous quantification of MDPV and two metabolites in plasma with good selectivity and sensitivity.
Keywords: HRMS; LC–MS/MS; MDPV; Metabolites; Synthetic cathinones.
Published by Elsevier B.V.
Figures
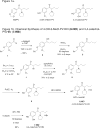
Figure 1
Figure 1a. Structures of 3,4-methylenedioxypyrovalerone (MDPV), 3,4-dihydroxypyrovalerone (3,4-Catechol-PV) and 4-hydroxy-3-methoxy-pyrovalerone (4-OH-3-MeO-PV). Figure 1b. Chemical Synthesis of 4-OH-3-MeO-PV.HCl (6.HCl) and 3,4-catechol-PV.HBr (7.HBr)
Figure 2
Full product ion mass spectra of 3,4-Catechol-PV (A) and 4-OH-3-MeO-PV (B) at normalized collision energy (NCE) ramp 35–65.
Figure 2
Full product ion mass spectra of 3,4-Catechol-PV (A) and 4-OH-3-MeO-PV (B) at normalized collision energy (NCE) ramp 35–65.
Figure 3
LC-MS/MS chromatogram of (a) human blank plasma, (b) human plasma fortified at 0.250 μg L−1 3,4-methylenedioxypyrovalerone (MDPV), 3,4-dihydroxypyrovalerone (3,4-catechol-PV) and 4-hydroxy-3-methoxypyrovalerone (4-OH-3-MeO-PV) and (c) a rat plasma specimen collected 240 min after 0.5 mg kg−1 intraperitoneal MDPV.
Similar articles
- Linear pharmacokinetics of 3,4-methylenedioxypyrovalerone (MDPV) and its metabolites in the rat: relationship to pharmacodynamic effects.
Anizan S, Concheiro M, Lehner KR, Bukhari MO, Suzuki M, Rice KC, Baumann MH, Huestis MA. Anizan S, et al. Addict Biol. 2016 Mar;21(2):339-47. doi: 10.1111/adb.12201. Epub 2014 Dec 5. Addict Biol. 2016. PMID: 25475011 Free PMC article. - Neuropharmacology of 3,4-Methylenedioxypyrovalerone (MDPV), Its Metabolites, and Related Analogs.
Baumann MH, Bukhari MO, Lehner KR, Anizan S, Rice KC, Concheiro M, Huestis MA. Baumann MH, et al. Curr Top Behav Neurosci. 2017;32:93-117. doi: 10.1007/7854_2016_53. Curr Top Behav Neurosci. 2017. PMID: 27830575 Free PMC article. Review. - Pharmacology of novel synthetic stimulants structurally related to the "bath salts" constituent 3,4-methylenedioxypyrovalerone (MDPV).
Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH. Marusich JA, et al. Neuropharmacology. 2014 Dec;87:206-13. doi: 10.1016/j.neuropharm.2014.02.016. Epub 2014 Mar 2. Neuropharmacology. 2014. PMID: 24594476 Free PMC article. - Neurobiology of 3,4-methylenedioxypyrovalerone (MDPV) and α-pyrrolidinovalerophenone (α-PVP).
Glennon RA, Young R. Glennon RA, et al. Brain Res Bull. 2016 Sep;126(Pt 1):111-126. doi: 10.1016/j.brainresbull.2016.04.011. Epub 2016 Apr 29. Brain Res Bull. 2016. PMID: 27142261 Free PMC article. Review.
Cited by
- Pharmacological mechanisms underlying the cardiovascular effects of the "bath salt" constituent 3,4-methylenedioxypyrovalerone (MDPV).
Schindler CW, Thorndike EB, Suzuki M, Rice KC, Baumann MH. Schindler CW, et al. Br J Pharmacol. 2016 Dec;173(24):3492-3501. doi: 10.1111/bph.13640. Epub 2016 Nov 16. Br J Pharmacol. 2016. PMID: 27714779 Free PMC article. - 3,4-Methylenedioxypyrovalerone (MDPV) Sensing Based on Electropolymerized Molecularly Imprinted Polymers on Silver Nanoparticles and Carboxylated Multi-Walled Carbon Nanotubes.
Couto RAS, Coelho C, Mounssef B Jr, Morais SFA, Lima CD, Dos Santos WTP, Carvalho F, Rodrigues CMP, Braga AAC, Gonçalves LM, Quinaz MB. Couto RAS, et al. Nanomaterials (Basel). 2021 Feb 1;11(2):353. doi: 10.3390/nano11020353. Nanomaterials (Basel). 2021. PMID: 33535439 Free PMC article. - Quantification of Synthetic Cathinones in Rat Brain Using HILIC-ESI-MS/MS.
Peters JR, Keasling R, Brown SD, Pond BB. Peters JR, et al. J Anal Toxicol. 2016 Nov;40(9):718-725. doi: 10.1093/jat/bkw074. Epub 2016 Jul 29. J Anal Toxicol. 2016. PMID: 27474358 Free PMC article. - Comprehensive review of the detection methods for synthetic cannabinoids and cathinones.
Namera A, Kawamura M, Nakamoto A, Saito T, Nagao M. Namera A, et al. Forensic Toxicol. 2015;33(2):175-194. doi: 10.1007/s11419-015-0270-0. Epub 2015 Mar 6. Forensic Toxicol. 2015. PMID: 26257831 Free PMC article. - Pharmacokinetic data of synthetic cathinones in female Sprague-Dawley rats.
Grecco GG, Kisor DF, Sprague JE. Grecco GG, et al. Data Brief. 2018 Oct 25;21:1045-1050. doi: 10.1016/j.dib.2018.10.073. eCollection 2018 Dec. Data Brief. 2018. PMID: 30450398 Free PMC article.
References
- Emerson TS, Cisek JE. Methcathinone: a Russian designer amphetamine infiltrates the rural midwest. Annals of Emergency Medicine. 1993;22:1897–1903. - PubMed
- Kelly JP. Cathinone derivatives: a review of their chemistry, pharmacology and toxicology. Drug Test Anal. 2011;3(7–8):439–453. - PubMed
- Kesha K, Boggs CL, Ripple MG, Allan CH, Levine B, Jufer-Phipps R, Doyon S, Chi P, Fowler DR. Methylenedioxypyrovalerone (“Bath Salts”),Related Death: Case Report and Review of the Literature. J Forensic Sci. 2013 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials