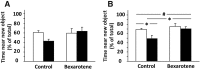Reversal of apoE4-driven brain pathology and behavioral deficits by bexarotene - PubMed (original) (raw)
Reversal of apoE4-driven brain pathology and behavioral deficits by bexarotene
Anat Boehm-Cagan et al. J Neurosci. 2014.
Abstract
Apolipoprotein E4 (apoE4), the most prevalent genetic risk factor for Alzheimer's disease (AD), is less lipidated than its corresponding AD-benign form, apoE3, and it has been suggested that the pathological effects of apoE4 are mediated by lipid-related mechanisms. ATP-binding cassette transporters A1 and G1 (ABCA1 and ABCG1, respectively) are the most important apoE-lipidating proteins. The expression of these proteins, as well as that of apoE, is controlled by the transcription regulation retinoid X receptor (RXR)-liver X receptor (LXR) system. In the present study, we investigated the effects of the RXR agonist bexarotene on mRNA and protein levels of apoE, ABCA1, and ABCG1 in young, naive apoE3- and apoE4-targeted replacement mice and assessed the extent to which this reverses the apoE4-driven pathological phenotype. This investigation reveled that bexarotene increases the mRNA and protein levels of ABCA1 and ABCG1 in hippocampal neurons, but has no effect on the corresponding levels of apoE. These findings were associated with reversal of the lipidation deficiency of apoE4 and of the cognitive impairments of apoE4 mice in several tests. Furthermore, bexarotene reversed the apoE4-driven accumulation of Aβ42 and hyperphosphorylated tau in hippocampal neurons, as well as the apoE4-induced reduction in the levels of the presynaptic marker vesicular glutamatergic transporter 1 (VGluT1). In conclusion, the results show that treatment of apoE4 mice with the RXR agonist bexarotene reverses the apoE4-induced cognitive and neuronal impairments in vivo and suggest that this is due to reversal of the lipidation deficiency of apoE4. This puts forward the possibility that RXR activation and increased levels of ABCA1 and ABCG1 could be useful in the treatment of human apoE4 carriers.
Keywords: ABCA1; ABCG1; Alzheimer's disease; ApoE4; apoE-targeted mice; bexarotene.
Copyright © 2014 the authors 0270-6474/14/347293-09$15.00/0.
Figures
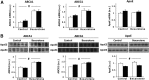
Figure 1.
The effect of bexarotene on the mRNA and protein levels of apoE, ABCA1, and ABCG1 in hippocampal neurons of apoE3 and apoE4 mice. A, qRT-PCR measurements of the mRNA levels of ABCA1, ABCG1, and apoE mice. The experiments were performed as described in Materials and Methods and the results are normalized relative to control apoE3 mice (mean ± SEM; n = 5 per group). White bars correspond to apoE3 mice, whereas black bars correspond to apoE4 mice. B, Immunoblot assays of the corresponding protein levels of ABCA1, ABCG1, and apoE mice. Whole hippocampi from control and bexarotene apoE3 and apoE4 mice were subjected to immunoblot assays using anti-apoE, anti-ABCG1, and anti-ABCA1 Abs as described in Materials and Methods. The results are normalized relative to control apoE3 mice (mean ± SEM; n = 10 per group). #p = 0.0001 by 2-way ANOVA for the effect of treatment on ABCA1 and ABCG1 protein levels and on ABCA1 mRNA levels and #p = 0.001 for the effect of treatment on the ABCG1 mRNA levels (F = 148.4, F = 48.06 for ABCA1 and ABCG1 protein levels and F = 17.82 and F = 17.02 for ABCA1 and ABCG1 mRNA levels, respectively). #p = 0.0001 (F = 45.79) for the effect of genotype on apoE protein levels by 2-way ANOVA.
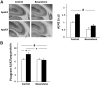
Figure 2.
The effect of bexarotene on apoE lipidation in the hippocampus of apoE3 and apoE4 mice. Freshly excised hippocampi from control and bexarotene-treated apoE4 and apoE3 mice were subjected to a nondenaturing 3–16% gradient gel and blotted using anti-apoE Ab, as described in Materials and Methods. As can be seen, the levels of apoE reactivity are lower in the control apoE4 mice compared with the control apoE3 mice and bexarotene treatment causes a significant increase in the appearance of high-molecular-weight species of apoE in both apoE4 and apoE3 mice.
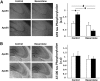
Figure 3.
Effect of bexarotene on the levels of Aβ42 in the hippocampus of apoE3 and apoE4 mice. A, Aβ42 immunohistochemistry. Brains of control and bexarotene-treated apoE4 and apoE3 mice were sectioned and subjected to histological staining with anti-Aβ42 Ab. Representative images (10× magnification) of the CA3 hippocampal field stained with anti-Aβ42 are presented on the left (scale bar, 200 μm) and show that the higher levels of Aβ42 in control apoE4 mice relative to the apoE3 mice are reduced in both apoE3 and apoE4 mice. Quantification of the results (mean ± SEM; n = 12–14 per group) of apoE3 mice (white bars) and apoE4 mice (black bars) was performed by computerized image analysis and is shown on the right. The experiment and data analysis were performed as described in Materials and Methods. The results are normalized relative to control apoE3 mice. #p < 0.001 (F = 41.61) for the effect of treatment by 2-way ANOVA. B, Aβ42 ELISA. Whole hippocampi from control and bexarotene-treated apoE4 and their corresponding apoE3 mice were subjected to Aβ X-42 ELISA kit, as described in Materials and Methods. The results (mean ± SEM; n = 5 per group) are normalized relative to control apoE3 mice and show a higher level of total Aβ42 in the control apoE4 mice, which is counteracted by the bexarotene treatment. #p < 0.05 (F = 7.86) for the effect of genotype × treatment by 2-way ANOVA; *p < 0.05 for the post hoc comparison of control apoE3 and apoE4 and for the effect of bexarotene on apoE4 mice by Tukey post hoc analysis.
Figure 4.
The effect of bexarotene on the levels of hyperphosphorylated tau in CA3 neurons of apoE3 and apoE4 mice. A, Effect of bexarotene on AT8 tau phosphorylation. Brains of control and bexarotene-treated apoE4 and apoE3 mice were sectioned and subjected to histological staining using AT8, a marker for tau phosphorylation (Ser202/Thr205). Representative images (10× magnification) of the CA3 hippocampal subfield are presented on the left (scale bar, 200 μm) and show that the increased level of tau phosphorylation in the apoE4 mice compared with the apoE3 mice is abolished by bexarotene. Quantification of the results (mean ± SEM; n = 12–14 per group) of apoE3 mice (white bars) and apoE4 mice (black bars) was performed by computerized image analysis, as described in Materials and Methods, and is shown on the right. The results are normalized to control apoE3 mice. #p < 0.001 (F = 23.2 and F = 15) for the effect of treatment and of genotype × treatment by 2-way ANOVA; *p < 0.001 for the post hoc comparison of control apoE3 and apoE4 and for the effect of bexarotene on apoE4 mice by Tukey post hoc analysis. B, Effect of bexarotene on AT100 tau phosphorylation. Experiments were performed and are depicted as described above. As can be seen, the results show no effect for genotype or treatment.
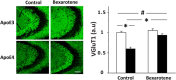
Figure 5.
Effect of bexarotene on the levels of the glutamate vesicular transporter VGluT1 in CA3 neurons of apoE3 and apoE4 mice. Brains of control and bexarotene-treated apoE4 mice and their corresponding apoE3 mice were sectioned and subjected to histological staining using anti-VGluT1 Ab. Representative images (20× magnification) of the CA3 hippocampal subfield are presented on the left (scale bar, 80 μm) and show that the decreased levels in the control apoE4 mice relative to the apoE3 mice are increased by bexarotene. Quantification of the results (mean ± SEM: n = 12–14 per group) of apoE3 mice (white bars) and apoE4 mice (black bars) was performed by computerized image analysis, as described in Materials and Methods, and is shown on the right. Results are normalized to control apoE3 mice. #p < 0.001 (F = 41.65, F = 22.58 and F = 12.4) for the effect of genotype, treatment and genotype × treatment by 2-way ANOVA; *p < 0.001 for the post hoc comparison of control apoE3 and apoE4 and for the effect of bexarotene on apoE4 mice by Tukey post hoc analysis.
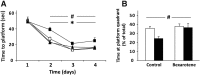
Figure 6.
Effect of bexarotene on the performance of apoE3 and apoE4 mice in the Morris water maze. Control and bexarotene apoE4 mice and their corresponding apoE3 mice were subjected to a Morris water maze test. A, Latency to reach the platform. Latency was tested across 4 daily trials for 4 d, as described in Materials and Methods. The results shown are the average latencies of the 4 daily trials of each group in seconds (n = 12–14 per group). □ and ■ correspond to control apoE3 and apoE4 mice, respectively. Δ and ▴ correspond to bexarotene-treated apoE3 and apoE4 mice, respectively. # p < 0.05 (F = 2.86) for the effect of genotype × treatment by 2-way ANOVA; *p < 0.05 for the effect of bexarotene on apoE4 mice by Tukey post hoc analysis. B, Probe test. The hidden platform was removed from the arena and the time the mice spent in the quadrant in which the platform was located was measured, as described in Materials and Methods. The results are depicted as the percentage of time spent in the platform's quadrant out of the total trial time (n = 6–7 per group). White bars correspond to apoE3 mice, whereas black bars correspond to apoE4 mice. #p < 0.05 (F = 5.47) for the effect of treatment by 2-way ANOVA.
Figure 7.
Effect of bexarotene on the performance of the apoE3 and apoE4 mice in the novel object recognition test. Control and bexarotene-treated apoE4 mice and corresponding apoE3 mice were first exposed to two identical objects (training session). This was followed by a delay of either 2 or 24 h, after which the mice were exposed to an old and a new object. The preference of the mice to the different objects was monitored, as described in Materials and Methods. A, Short-term memory test performed 2 h after the training session. B, Long-term memory test performed 24 h after the training session. The results obtained are presented as the percentage of time the mice spent near the novel object out of the total time spent near both old and novel objects. White bars correspond to apoE3 mice, whereas black bars correspond to apoE4 mice (n = 6–8 per group). #p < 0.05 (F = 5.13) for the effect of genotype × treatment by 2-way ANOVA; *p < 0.05 for the post hoc comparison of control apoE3 and apoE4 and for the effect of bexarotene on apoE4 mice by Tukey post hoc analysis.
Similar articles
- ATP-binding cassette transporter A1: from metabolism to neurodegeneration.
Koldamova R, Fitz NF, Lefterov I. Koldamova R, et al. Neurobiol Dis. 2014 Dec;72 Pt A:13-21. doi: 10.1016/j.nbd.2014.05.007. Epub 2014 May 17. Neurobiol Dis. 2014. PMID: 24844148 Free PMC article. Review. - Amyloid-β pathology and APOE genotype modulate retinoid X receptor agonist activity in vivo.
Tai LM, Koster KP, Luo J, Lee SH, Wang YT, Collins NC, Ben Aissa M, Thatcher GRJ, LaDu MJ. Tai LM, et al. J Biol Chem. 2014 Oct 31;289(44):30538-30555. doi: 10.1074/jbc.M114.600833. Epub 2014 Sep 12. J Biol Chem. 2014. PMID: 25217640 Free PMC article. - Retinoic acid isomers facilitate apolipoprotein E production and lipidation in astrocytes through the retinoid X receptor/retinoic acid receptor pathway.
Zhao J, Fu Y, Liu CC, Shinohara M, Nielsen HM, Dong Q, Kanekiyo T, Bu G. Zhao J, et al. J Biol Chem. 2014 Apr 18;289(16):11282-11292. doi: 10.1074/jbc.M113.526095. Epub 2014 Mar 5. J Biol Chem. 2014. PMID: 24599963 Free PMC article. - ABCA1 Agonist Reverses the ApoE4-Driven Cognitive and Brain Pathologies.
Boehm-Cagan A, Bar R, Liraz O, Bielicki JK, Johansson JO, Michaelson DM. Boehm-Cagan A, et al. J Alzheimers Dis. 2016 Oct 4;54(3):1219-1233. doi: 10.3233/JAD-160467. J Alzheimers Dis. 2016. PMID: 27567858 - The role of ATP-binding cassette transporter G1 (ABCG1) in Alzheimer's disease: A review of the mechanisms.
Yazdi MK, Alavi MS, Roohbakhsh A. Yazdi MK, et al. Basic Clin Pharmacol Toxicol. 2024 Apr;134(4):423-438. doi: 10.1111/bcpt.13981. Epub 2024 Jan 26. Basic Clin Pharmacol Toxicol. 2024. PMID: 38275217 Review.
Cited by
- The Role of APOE and TREM2 in Alzheimer's Disease-Current Understanding and Perspectives.
Wolfe CM, Fitz NF, Nam KN, Lefterov I, Koldamova R. Wolfe CM, et al. Int J Mol Sci. 2018 Dec 26;20(1):81. doi: 10.3390/ijms20010081. Int J Mol Sci. 2018. PMID: 30587772 Free PMC article. Review. - ATP-binding cassette transporter A1: from metabolism to neurodegeneration.
Koldamova R, Fitz NF, Lefterov I. Koldamova R, et al. Neurobiol Dis. 2014 Dec;72 Pt A:13-21. doi: 10.1016/j.nbd.2014.05.007. Epub 2014 May 17. Neurobiol Dis. 2014. PMID: 24844148 Free PMC article. Review. - Protein misfolding in neurodegenerative diseases: implications and strategies.
Sweeney P, Park H, Baumann M, Dunlop J, Frydman J, Kopito R, McCampbell A, Leblanc G, Venkateswaran A, Nurmi A, Hodgson R. Sweeney P, et al. Transl Neurodegener. 2017 Mar 13;6:6. doi: 10.1186/s40035-017-0077-5. eCollection 2017. Transl Neurodegener. 2017. PMID: 28293421 Free PMC article. Review. - Interactions between inflammation, sex steroids, and Alzheimer's disease risk factors.
Uchoa MF, Moser VA, Pike CJ. Uchoa MF, et al. Front Neuroendocrinol. 2016 Oct;43:60-82. doi: 10.1016/j.yfrne.2016.09.001. Epub 2016 Sep 17. Front Neuroendocrinol. 2016. PMID: 27651175 Free PMC article. Review. - New insights in lipid metabolism: potential therapeutic targets for the treatment of Alzheimer's disease.
Cao Y, Zhao LW, Chen ZX, Li SH. Cao Y, et al. Front Neurosci. 2024 Sep 11;18:1430465. doi: 10.3389/fnins.2024.1430465. eCollection 2024. Front Neurosci. 2024. PMID: 39323915 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous