Neuroprotection after traumatic brain injury in heat-acclimated mice involves induced neurogenesis and activation of angiotensin receptor type 2 signaling - PubMed (original) (raw)
Neuroprotection after traumatic brain injury in heat-acclimated mice involves induced neurogenesis and activation of angiotensin receptor type 2 signaling
Gali Umschweif et al. J Cereb Blood Flow Metab. 2014 Aug.
Abstract
Long-term exposure of mice to mild heat (34°C±1°C) confers neuroprotection against traumatic brain injury (TBI); however, the underling mechanisms are not fully understood. Heat acclimation (HA) increases hypothalamic angiotensin II receptor type 2 (AT2) expression and hypothalamic neurogenesis. Accumulating data suggest that activation of the brain AT2 receptor confers protection against several types of brain pathologies, including ischemia, a hallmark of the secondary injury occurring following TBI. As AT2 activates the same pro-survival pathways involved in HA-mediated neuroprotection (e.g., Akt phosphorylation, hypoxia-inducible factor 1α (HIF-1α), and brain-derived neurotrophic factor (BDNF)), we examined the role of AT2 in HA-mediated neuroprotection after TBI. Using an AT2-specific antagonist PD123319, we found that the improvements in motor and cognitive recovery as well as reduced lesion volume and neurogenesis seen in HA mice were all diminished by AT2 inhibition, whereas no significant alternations were observed in control mice. We also found that nerve growth factor/tropomyosin-related kinase receptor A (TrkA), BDNF/TrkB, and HIF-1α pathways are upregulated by HA and inhibited on PD123319 administration, suggesting that these pathways play a role in AT2 signaling in HA mice. In conclusion, AT2 is involved in HA-mediated neuroprotection, and AT2 activation may be protective and should be considered a novel drug target in the treatment of TBI patients.
Figures
Figure 1
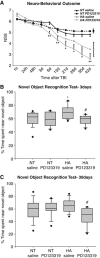
Heat acclimation (HA)-mediated neuroprotection and cognitive performance are compromised by angiotensin receptor type 2 blockade after traumatic brain injury (TBI). PD123319 (PD, 10 mg/kg/day) or saline-treated mice were subjected to TBI, and neurobehavioral outcome was evaluated by the Neurological Severity Score (NSS) at 1 hour post injury to assess initial disability and at multiple time points thereafter. PD treatment eliminated the statistically significant improvement in neurobehavioral outcome of HA mice (A; *P<0.05; **P<0.01 HA treated with saline versus normothermic (NT) treated with saline, determined by two-way analysis of variance for repeated measures, _n_=9 to 10 per group). For the sake of clarity, maximal error bars of the groups treated with PD123319 are presented on the right end of the representative line. In the box plots, mice were subjected to the novel object recognition test (NORT) 3 days (B) and 30 days (C) after TBI. The graph shows the median (band), 25th and 75th quantiles (bottom and top of box), sample minimum and maximum (whiskers), and outliers (dots). The longer exploration time of the novel object of HA mice decreased after PD123319 (PD, 10 mg/kg/day) treatment. *P<0.05 HA treated with saline versus NT treated with saline; #P<0.05 HA treated with saline versus HA treated with PD123319, determined by Mann–Whitney, _n_=9 to 10 per group.
Figure 2
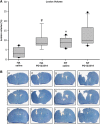
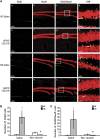
Heat acclimation (HA)-mediated reduced lesion volume is eliminated by angiotensin receptor type 2 blockade after traumatic brain injury (TBI). Mice were killed 42 days after TBI, and evenly separated 10-_μ_m slices were stained with Giemsa. (A) The graph shows the median (band), 25th and 75th quantiles (bottom and top of box), sample minimum and maximum (whiskers), and outliers (dots). (B) The reduced lesion volume measured in HA mice (panels a–c) was abolished by PD123319 treatment (panels d–f, PD123319, 10 mg/kg/day). *P<0.05 HA treated with saline versus normothermic (NT) treated with saline (panels g–i); #P<0.05 HA treated with saline versus HA treated with PD123319, as determined by Mann–Whitney (_n_=6 per group).
Figure 3
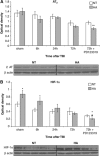
Angiotensin receptor type 2 (AT2) blockade decreases protein levels of AT2 and hypoxia-inducible factor 1_α_ (HIF-1_α_) in heat-acclimated (HA) mice. Mice were subjected to traumatic brain injury (TBI) after treatment with saline or PD123319 (PD, 10 mg/kg/day), and killed at 6, 24, or 72 hours post TBI. (A) HA did not alter AT2 levels; however, PD123319 treatment lowered AT2 levels in HA mice. (B) Elevated HIF-1_α_ levels were measured in HA mice and were reduced after PD123319 treatment. *P<0.05 between HA treated with saline versus normothermic (NT) treated with saline; **P<0.05 between HA treated with PD123319 versus NT treated with PD123319; #P<0.05 HA treated with saline versus HA treated with PD123319, determined by two-way analysis of variance followed by Tukey's test, _n_=6 per group.
Figure 4
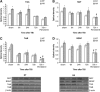
Angiotensin receptor type 2 is involved in heat acclimation (HA)-mediated enhanced neurotrophin signaling. Mice were subjected to traumatic brain injury (TBI) after treatment with saline or PD123319 (PD, 10 mg/kg/day), and were killed at 6, 24, or 72 hours post TBI. HA elevated levels of tropomyosin-related kinase receptor A (TrkA, A) as well as its endogenous ligand, nerve growth factor (NGF). PD123319 treatment decreased NGF levels in HA mice (B). Elevated TrkB levels were seen in HA mice and were reduced after PD123319 treatment in both normothermic (NT) and HA mice (C). HA induced brain-derived neurotrophic factor (BDNF) levels, whereas PD123319 treatment reduced BDNF levels, in both NT and HA mice (D). *P<0.05 HA treated with saline versus NT treated with saline; #P<0.05 mice treated with saline versus mice treated with PD123319 of the same group (NT/HA), determined by two-way analysis of variance followed by Tukey's test, _n_=6 per group.
Figure 5
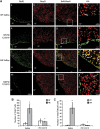
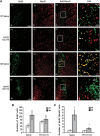
Angiotensin receptor type 2 is involved in heat acclimation (HA)-mediated enhanced neurogenesis in the subventricular zone (SVZ). Mice were subjected to traumatic brain injury (TBI) after treatment with saline or PD123319 (PD, 10 mg/kg/day) for 3 days, and killed 42 days post TBI. Evenly sliced 10-_μ_m sections were incubated with anti-NeuN for mature neurons and with anti-BrdU (anti-5-bromo-2-deoxyuridine) for newborn cells. (A) HA induced neurogenesis in the SVZ (i, j) and PD123319 (PD, 10 mg/kg/day) treatment blocked the observed neurogenesis (m–p). PD treatment (e–h) did not reduce neurogenesis in normothermic (NT) mice measured after saline treatment (a–d). Stereological quantification of the fields was used to count the number of BrdU-positive cells (BrdU+). Results show the average number of BrdU+ per field (B), and the average number of double-positive cells for NeuN and for BrdU (BrdU+/NeuN+) per field (C). *P<0.01 HA treated with saline versus NT treated with saline; #P<0.01 HA mice treated with saline versus HA mice treated with PD123319, determined by two-way analysis of variance followed by Tukey's test, _n_=6 per group.
Figure 6
Angiotensin receptor type 2 is involved in heat acclimation (HA)-mediated enhanced neurogenesis in the dentate gyrus. Mice were subjected to traumatic brain injury (TBI) and treated with saline or PD123319 (PD, 10 mg/kg/day) for 3 days, and killed 42 days post TBI. Evenly sliced 10-_μ_m sections were incubated with anti-NeuN for mature neurons and with anti-BrdU (anti-5-bromo-2-deoxyuridine) for newborn cells. (A) HA induced neurogenesis in the dentate gyrus subgranular zone (i, j) and PD123319 (PD, 10 mg/kg/day) treatment blocked the observed neurogenesis (m–p). PD treatment (e–h) did not reduce neurogenesis in normothermic (NT) mice (a–d). Stereological quantification of the fields was used to count the number of BrdU-positive cells (BrdU+). Results show the average number of BrdU+ per field (B), and the average number of double-positive cells for NeuN and for BrdU (BrdU+/NeuN+) per field (C). *P<0.05 HA treated with saline versus NT treated with saline; #P<0.05 HA mice treated with saline versus HA mice treated with PD123319, determined by two-way analysis of variance followed by Tukey's test, _n_=6 per group.
Figure 7
Angiotensin receptor type 2 is involved in heat acclimation (HA)-mediated enhanced neurogenesis in the injury region (inj). Mice were subjected to traumatic brain injury (TBI) and treated with saline or PD123319 (PD, 10 mg/kg/day) for 3 days, and killed 42 days post TBI. Evenly sliced 10-_μ_m sections were incubated with anti-NeuN for mature neurons and with anti-BrdU (anti-5-bromo-2-deoxyuridine) for newborn cells. (A) HA induced neurogenesis in the injury region (i, j) and PD123319 (PD, 10 mg/kg/day) treatment blocked the observed neurogenesis (m–p). PD treatment (e–h) did not reduce neurogenesis in normothermic (NT) mice (a–d). Stereological quantification of the fields was used to count the number of BrdU-positive cells (BrdU+). Results show the average number of BrdU+ per field (B), and the average number of double-positive cells for NeuN and for BrdU (BrdU+/NeuN+) per field (C). *P<0.01 HA treated with saline versus NT treated with saline; #P<0.01 HA mice treated with saline versus HA mice treated with PD123319, determined by two-way analysis of variance followed by Tukey's test, _n_=6 per group.
Similar articles
- Hypoxia-inducible factor 1 is essential for spontaneous recovery from traumatic brain injury and is a key mediator of heat acclimation induced neuroprotection.
Umschweif G, Alexandrovich AG, Trembovler V, Horowitz M, Shohami E. Umschweif G, et al. J Cereb Blood Flow Metab. 2013 Apr;33(4):524-31. doi: 10.1038/jcbfm.2012.193. Epub 2013 Jan 2. J Cereb Blood Flow Metab. 2013. PMID: 23281425 Free PMC article. - Angiotensin receptor type 2 activation induces neuroprotection and neurogenesis after traumatic brain injury.
Umschweif G, Liraz-Zaltsman S, Shabashov D, Alexandrovich A, Trembovler V, Horowitz M, Shohami E. Umschweif G, et al. Neurotherapeutics. 2014 Jul;11(3):665-78. doi: 10.1007/s13311-014-0286-x. Neurotherapeutics. 2014. PMID: 24957202 Free PMC article. - Heat acclimation increases hypoxia-inducible factor 1alpha and erythropoietin receptor expression: implication for neuroprotection after closed head injury in mice.
Shein NA, Horowitz M, Alexandrovich AG, Tsenter J, Shohami E. Shein NA, et al. J Cereb Blood Flow Metab. 2005 Nov;25(11):1456-65. doi: 10.1038/sj.jcbfm.9600142. J Cereb Blood Flow Metab. 2005. PMID: 15902197 - Molecular programs induced by heat acclimation confer neuroprotection against TBI and hypoxic insults via cross-tolerance mechanisms.
Horowitz M, Umschweif G, Yacobi A, Shohami E. Horowitz M, et al. Front Neurosci. 2015 Jul 28;9:256. doi: 10.3389/fnins.2015.00256. eCollection 2015. Front Neurosci. 2015. PMID: 26283898 Free PMC article. Review. - The angiotensin AT2 receptor in left ventricular hypertrophy.
Steckelings UM, Widdop RE, Paulis L, Unger T. Steckelings UM, et al. J Hypertens. 2010 Sep;28 Suppl 1:S50-5. doi: 10.1097/01.hjh.0000388495.66330.63. J Hypertens. 2010. PMID: 20823717 Review.
Cited by
- Cross-tolerance: embryonic heat conditioning induces inflammatory resilience by affecting different layers of epigenetic mechanisms regulating IL6 expression later in life.
Rosenberg T, Kisliouk T, Ben-Nun O, Cramer T, Meiri N. Rosenberg T, et al. Epigenetics. 2021 Jan-Feb;16(2):228-241. doi: 10.1080/15592294.2020.1795596. Epub 2020 Jul 24. Epigenetics. 2021. PMID: 32705933 Free PMC article. - Interaction between Angiotensin Type 1, Type 2, and Mas Receptors to Regulate Adult Neurogenesis in the Brain Ventricular-Subventricular Zone.
Garcia-Garrote M, Perez-Villalba A, Garrido-Gil P, Belenguer G, Parga JA, Perez-Sanchez F, Labandeira-Garcia JL, Fariñas I, Rodriguez-Pallares J. Garcia-Garrote M, et al. Cells. 2019 Nov 30;8(12):1551. doi: 10.3390/cells8121551. Cells. 2019. PMID: 31801296 Free PMC article. - The Role of BDNF in Experimental and Clinical Traumatic Brain Injury.
Gustafsson D, Klang A, Thams S, Rostami E. Gustafsson D, et al. Int J Mol Sci. 2021 Mar 30;22(7):3582. doi: 10.3390/ijms22073582. Int J Mol Sci. 2021. PMID: 33808272 Free PMC article. Review. - AT2 activation does not influence brain damage in the early phase after experimental traumatic brain injury in male mice.
Timaru-Kast R, Garcia Bardon A, Luh C, Coronel-Castello SP, Songarj P, Griemert EV, Krämer TJ, Sebastiani A, Steckelings UM, Thal SC. Timaru-Kast R, et al. Sci Rep. 2022 Aug 22;12(1):14280. doi: 10.1038/s41598-022-18338-x. Sci Rep. 2022. PMID: 35995819 Free PMC article. - Depletion of angiotensin-converting enzyme 2 reduces brain serotonin and impairs the running-induced neurogenic response.
Klempin F, Mosienko V, Matthes S, Villela DC, Todiras M, Penninger JM, Bader M, Santos RAS, Alenina N. Klempin F, et al. Cell Mol Life Sci. 2018 Oct;75(19):3625-3634. doi: 10.1007/s00018-018-2815-y. Epub 2018 Apr 20. Cell Mol Life Sci. 2018. PMID: 29679094 Free PMC article.
References
- Horowitz M. Heat acclimation, epigenetics, and cytoprotection memory. Compr Physiol. 2014;4:199–230. - PubMed
- Dumont EC, Rafrafi S, Laforest S, Drolet G. Involvement of central angiotensin receptors in stress adaptation. Neuroscience. 1999;93:877–884. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials