The complex ultrastructure of the endolysosomal system - PubMed (original) (raw)
Review
The complex ultrastructure of the endolysosomal system
Judith Klumperman et al. Cold Spring Harb Perspect Biol. 2014.
Abstract
Live-cell imaging reveals the endolysosomal system as a complex and highly dynamic network of interacting compartments. Distinct types of endosomes are discerned by kinetic, molecular, and morphological criteria. Although none of these criteria, or combinations thereof, can capture the full complexity of the endolysosomal system, they are extremely useful for experimental purposes. Some membrane domain specializations and specific morphological characteristics can only be seen by ultrastructural analysis after preparation for electron microscopy (EM). Immuno-EM allows a further discrimination of seemingly identical compartments by their molecular makeup. In this review we provide an overview of the ultrastructural characteristics and membrane organization of endosomal compartments, along with their organizing machineries.
Copyright © 2014 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
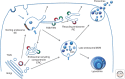
Figure 1.
Schematic and simplified representation of the endolysosomal system showing the different organelles described in this article. Sorting endosomes (SE) are vacuolar compartments often bearing bilayered, flat clathrin coats (brown). Tubules emanate from SE that form the recycling endosomes (RE). The RE may localize to the _peri_-Golgi area forming the endocytic recycling compartment (ERC) or distribute to the cell periphery. The RE network is complex with multiple sorting sites, thereby the tubular sorting endosome (TSE) or tubular endosomal network (TEN) is also represented. AP1 (red) and AP3 (green) coated buds on RE (ERC/TSE/TEN) are shown. Late endosomes correspond to multivesicular bodies (MVBs) filled with intraluminal vesicles (ILVs). MVBs are fated for fusion with lysosomes. In some cells, a population of MVBs fuse with the plasma membrane, a process during which the ILVs are secreted as exosomes. Gray arrows indicate directions of transport/maturation of compartments. Blue arrows indicate invagination at the endosomal membrane of SE and MVBs required for ILV formation.
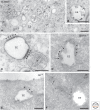
Figure 2.
Picture gallery of early endosomes. (A) HepG2 cells pre-incubated for 3 h with BSA conjugated to 5-nm gold (BSA5). Both early endosomes (EEs) and late endosomes (LEs) are seen. EEs have an electron lucent content with some intraluminal vesicles (ILVs). LEs contain multiple ILVs. (B, inset) An enlargement of an EE clearly showing that internalized BSA5 is not present in ILVs, which contain cytosolic content. (C) Melanocytic cell line analyzed by conventional electron microscopy. Bi-layered coats (arrowheads) are abundant on EEs. Note their typical appearance with an electron-dense layer lining the endosomal membrane and the fuzzier, clathrin-containing layer facing the cytoplasm. (*, on the right) A pre-melanosome with intraluminal fibrils. (D) EE from a CHO cell immunogold-labeled for clathrin (10-nm gold). Labeling is present on the coated areas of the limiting membrane (arrowheads). Budding of ILVs occurs at the edges of the coat (dark arrows). (White arrow) Points to a recycling tubule extending from the uncoated region of the endosomal membrane. (Adapted from Sachse et al. 2002b, with permission from the author.) (E) Internalized growth hormone (10-nm gold) selectively localizes to the part of the endosomal membrane that is covered by the clathrin-coat (arrowheads). This EE does not yet contain ILVs. (From Sachse et al. 2002a; adapted, with permission, from the author.) (F) EE in brown adipose tissue immunogold-labeled for albumin (5-nm gold) and Glucose Transporter 4 (10-nm gold). Note the separation of albumin label, in the EE vacuole, and GT label in the emerging recycling tubule (arrows). (Based on Slot et al. 1991; courtesy of Hans Geuze.) Techniques used: (A,B) HPF and EPON embedding; (C) chemical fixation and EPON embedding; (D–F) chemical fixation and ultrathin cryosectioning. P, Plasma membrane. Scale bar, 200 nm.
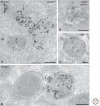
Figure 3.
Picture gallery of late endosomes. (A) HepG2 cells pre-incubated for 3 h with BSA5 and immunogold-labeled for LAMP2 (15-nm gold particles). LE vacuoles contain multiple ILVs but can differ in size and shape. Note the recycling tubule emerging from the LE (arrow). The lysosome (L) has not been reached by BSA5. (B) MVB/MHC class II compartment of a dendritic cell immunogold-labeled for MHC class II (15-nm gold) and CD63 (10-nm gold). Note that the ILVs are heterogeneous in size. CD63 is rather associated with smaller vesicles, whereas MHC class II is present on the larger vesicles and also with the limiting membrane. (C) Typical example of an LE/MVB. Immunogold labeling for LAMP1 (10-nm gold) is mainly restricted to the limiting membrane. Note the different morphological features of the ILVs present in these distinct MVBs (A–C). (D) HepG2 cells pre-incubated for 3 h with BSA5. Fusion profile (arrow) of an LE positive for BSA5 with a lysosome devoid of BSA5. Techniques used: (A–C) Chemical fixation and ultrathin cryosectioning; (D) HPF, rehydration, ultrathin cryosectioning. Scale bar, 200 nm.
Figure 4.
Picture gallery of lysosomes. (A) HepG2 cells pre-incubated for 3 h with BSA5 and immunogold-labeled for LAMP1 (10-nm gold). Example of a typical lysosome with lamellar membranes, some ILVs, and an overall electron-dense content. LAMP1 is mainly restricted to the limiting membrane. (B) Another example from a HepG2 cell, showing the presence of lysosomal enzyme cathepsin B (CatB) (15-nm gold) in the electron-dense lumen and LAMP1 (10-nm gold) at the limiting membrane. (C) Autophagic compartments in a stimulated B cell. The asterisk points to a double-membrane vesicle sequestering the cytosol. The autolysome (AL) contains more heterogeneous content than a regular lysosome. Techniques used: (A,B) chemical fixation and ultrathin cryosectioning; (C) chemical fixation and EPON embedding. Scale bar, 200 nm.
Similar articles
- Quantitative correlative microscopy reveals the ultrastructural distribution of endogenous endosomal proteins.
van der Beek J, de Heus C, Liv N, Klumperman J. van der Beek J, et al. J Cell Biol. 2022 Jan 3;221(1):e202106044. doi: 10.1083/jcb.202106044. Epub 2021 Nov 24. J Cell Biol. 2022. PMID: 34817533 Free PMC article. - Role of recycling endosomes and lysosomes in dynein-dependent entry of canine parvovirus.
Suikkanen S, Sääjärvi K, Hirsimäki J, Välilehto O, Reunanen H, Vihinen-Ranta M, Vuento M. Suikkanen S, et al. J Virol. 2002 May;76(9):4401-11. doi: 10.1128/jvi.76.9.4401-4411.2002. J Virol. 2002. PMID: 11932407 Free PMC article. - Taxol inhibits endosomal-lysosomal membrane trafficking at two distinct steps in CV-1 cells.
Sonee M, Barrón E, Yarber FA, Hamm-Alvarez SF. Sonee M, et al. Am J Physiol. 1998 Dec;275(6):C1630-9. doi: 10.1152/ajpcell.1998.275.6.C1630. Am J Physiol. 1998. PMID: 9843725 - ER contact sites direct late endosome transport.
Wijdeven RH, Jongsma ML, Neefjes J, Berlin I. Wijdeven RH, et al. Bioessays. 2015 Dec;37(12):1298-302. doi: 10.1002/bies.201500095. Epub 2015 Oct 6. Bioessays. 2015. PMID: 26440125 Review. - Endocytosis and exocytosis: current concepts of vesicle traffic in animal cells.
Willingham MC, Pastan I. Willingham MC, et al. Int Rev Cytol. 1984;92:51-92. doi: 10.1016/s0074-7696(08)61324-8. Int Rev Cytol. 1984. PMID: 6150907 Review.
Cited by
- Activated Protein Kinase C (PKC) Is Persistently Trafficked with Epidermal Growth Factor (EGF) Receptor.
Heckman CA, Biswas T, Dimick DM, Cayer ML. Heckman CA, et al. Biomolecules. 2020 Sep 7;10(9):1288. doi: 10.3390/biom10091288. Biomolecules. 2020. PMID: 32906765 Free PMC article. - PI4P and BLOC-1 remodel endosomal membranes into tubules.
Jani RA, Di Cicco A, Keren-Kaplan T, Vale-Costa S, Hamaoui D, Hurbain I, Tsai FC, Di Marco M, Macé AS, Zhu Y, Amorim MJ, Bassereau P, Bonifacino JS, Subtil A, Marks MS, Lévy D, Raposo G, Delevoye C. Jani RA, et al. J Cell Biol. 2022 Nov 7;221(11):e202110132. doi: 10.1083/jcb.202110132. Epub 2022 Sep 28. J Cell Biol. 2022. PMID: 36169638 Free PMC article. - Understanding intracellular nanoparticle trafficking fates through spatiotemporally resolved magnetic nanoparticle recovery.
Sheridan E, Vercellino S, Cursi L, Adumeau L, Behan JA, Dawson KA. Sheridan E, et al. Nanoscale Adv. 2021 Mar 3;3(9):2397-2410. doi: 10.1039/d0na01035a. eCollection 2021 May 4. Nanoscale Adv. 2021. PMID: 36134166 Free PMC article. Review. - Molecular identification of a BAR domain-containing coat complex for endosomal recycling of transmembrane proteins.
Simonetti B, Paul B, Chaudhari K, Weeratunga S, Steinberg F, Gorla M, Heesom KJ, Bashaw GJ, Collins BM, Cullen PJ. Simonetti B, et al. Nat Cell Biol. 2019 Oct;21(10):1219-1233. doi: 10.1038/s41556-019-0393-3. Epub 2019 Oct 1. Nat Cell Biol. 2019. PMID: 31576058 Free PMC article. - Electrophysiological Techniques on the Study of Endolysosomal Ion Channels.
Chen CC. Chen CC. Handb Exp Pharmacol. 2023;278:217-233. doi: 10.1007/164_2023_638. Handb Exp Pharmacol. 2023. PMID: 36871125
References
- Allen RD, Fok AK 1980. Membrane recycling and endocytosis in Paramecium confirmed by horseradish peroxidase pulse-chase studies. J Cell Sci 45: 131–145 - PubMed
- Almeida CG, Yamada A, Tenza D, Louvard D, Raposo G, Coudrier E 2011. Myosin 1b promotes the formation of post-Golgi carriers by regulating actin assembly and membrane remodelling at the trans-Golgi network. Nat Cell Biol 13: 779–789 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources