The intersection of amyloid beta and tau at synapses in Alzheimer's disease - PubMed (original) (raw)
Review
The intersection of amyloid beta and tau at synapses in Alzheimer's disease
Tara L Spires-Jones et al. Neuron. 2014.
Abstract
The collapse of neural networks important for memory and cognition, including death of neurons and degeneration of synapses, causes the debilitating dementia associated with Alzheimer's disease (AD). We suggest that synaptic changes are central to the disease process. Amyloid beta and tau form fibrillar lesions that are the classical hallmarks of AD. Recent data indicate that both molecules may have normal roles at the synapse, and that the accumulation of soluble toxic forms of the proteins at the synapse may be on the critical path to neurodegeneration. Further, the march of neurofibrillary tangles through brain circuits appears to take advantage of recently described mechanisms of transsynaptic spread of pathological forms of tau. These two key phenomena, synapse loss and the spread of pathology through the brain via synapses, make it critical to understand the physiological and pathological roles of amyloid beta and tau at the synapse.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
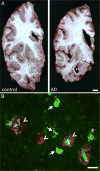
Figure 1. Neuropathology of AD
AD brains are characterized by striking atrophy compared to control brains (A). Particularly evident is shrinkage of the cortical mantle and the hippocampus (asterisk shows hippocampal atrophy). Microscopically, AD is defined by deposition of Aβ in senile plaques (arrowheads) and tau in neurofibrillary tangles (arrows). In this micrograph, the fibrillar deposits (both plaques and tangles) are stained green with thioflavine S. Aβ is also immunostained with antibody AW7 (courtesy Dominic Walsh), illustrating the halo of soluble Aβ around fibrillar plaque cores and the heterogeneous nature of plaques. Scale bars represent 1 cm (A) and 20 μm (B).
Figure 2. Structural changes in AD brain
The neural circuitry involved in memory including the entorhinal cortex-hippocampal circuitry (A) are severely affected by AD pathology including the deposition of plaques (blue) and tangles (green) and dramatic neuronal and synapse loss. Along with the dramatic neuronal loss, there are structural changes to remaining neurons in the AD brain that are thought to contribute to neural circuit disruption and cognitive impairments (B), including damage to neurites in the halo of soluble amyloid beta surrounding plaques, tau aggregation in cell bodies and neurites, and synapse loss associated with oligomeric Aβ around plaques. Panel A modified from (Gomez-Isla et al., 2008).
Figure 3. Dendritic spine loss in AD mouse models
Mouse models that exhibit plaque formation or tangle formation exhibit dendritic spine loss. Crossing APP/PS1 mice (A) and rTg4510 mice (B) with YFP overexpressing lines allowed quantification of dendritic spine density on cortical pyramidal neurons (layer II/III). Dense plaques are stained with thioflavine S in (A) and neurofibrillary tangles are stained with PHF1 antibody in B, while neurons in both panels are filled with YFP due to transgenic overexpression. Similar results are found when fluorescent markers are introduced via viral infection of neurons or direct injection of fluorophores. In plaque bearing mice, dendritic spine loss is most pronounced within 50 μm of plaques, whereas in tangle bearing mice, the presence of a tangle does not affect dendritic spine density (C). Data in C adapted from (Kopeikina et al., 2012b; Kopeikina et al., 2013; Rocher et al., 2010; Rozkalne et al., 2011; Spires et al., 2005). Scale bars represent 20 μm (A) and 50 μm (B).
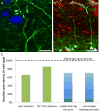
Figure 4
Array tomography reveals colocalization of oligomeric Aβ with synapses in human brain. The array tomography technique overcomes the axial resolution of light microscopy by physically sectioning resin embedded brain tissue into ribbons of ultrathin (70nm) serial sections which are stained with immunofluorescence, imaged with a fluorescent microscope at the same place along the ribbon (red dots) and a three dimensional dataset acquired of multiple markers at synapses (A, D). Using human AD brain tissue (B–C), we observed oligomeric Aβ stained with NAB61 (red) present at a subset of synapses as can be seen in the inset in panel B (presynaptic terminals stained here with synapsin I, green). We also observe a reduction in synapse density in the halo of oligomeric Aβ surrounding the Thioflavin S (ThioS) positive dense cores of plaques (arrows). Scale bars represent 5 μm (B, C) and 1 μm (inset in B). Panel D is a reconstruction of a 36 μm × 33 μm × 1.2 μm volume (images from 17 serial sections). Panel A adapted from Micheva and Smith 2007.
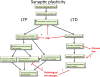
Figure 5. Pathways involved in normal synaptic plasticity and how they may be affected in AD
Under normal conditions, LTP promotes recruitment of neurotransmitter receptors to active synapses and causes synapse potentiation, stabilization, and growth. LTD conversely results in synapse depotentiation and spine collapse. Both of these processes are affected in animal models of AD with oligomeric Aβ clearly affecting the calcium and calcineurin pathways involved in these phenomena. Tau overexpression has been observed to affect synaptic function in transgenic models and to be necessary for oligomeric Aβ mediated synapse dysfunction, but the mechanisms by which pathological forms of tau affect synaptic plasticity are less well understood. It is possible that hyperphosphorylation affects microtubule stability and the transport of mitochondria to synapses which could affect synaptic function. The cleavage of tau by caspase 3 has also been observed which could be tied to the non-apoptotic role of caspase 3 in LTD and spine collapse.
Figure 6. Synaptic effects of Aβ and tau
Many studies implicate oligomeric Aβ in synapse dysfunction and loss in models of AD. Aβ may be specifically trafficked to the synapse by apoE4, where it binds to postsynaptic receptors, causes an increase in calcium concentration, calcineurin activation, caspase 3 activation, and downstream internalization of synaptic receptors. Tau has also been implicated in synapse dysfunction downstream of Aβ, and pathological forms of tau (pTau) are transferred through synaptic circuits, although which forms of tau are transported and how they are transported remains to be determined. Figure courtesy A Hermann.
Similar articles
- The intersection of amyloid β and tau in glutamatergic synaptic dysfunction and collapse in Alzheimer's disease.
Crimins JL, Pooler A, Polydoro M, Luebke JI, Spires-Jones TL. Crimins JL, et al. Ageing Res Rev. 2013 Jun;12(3):757-63. doi: 10.1016/j.arr.2013.03.002. Epub 2013 Mar 22. Ageing Res Rev. 2013. PMID: 23528367 Free PMC article. Review. - Synaptic Mitochondria: An Early Target of Amyloid-β and Tau in Alzheimer's Disease.
Torres AK, Jara C, Park-Kang HS, Polanco CM, Tapia D, Alarcón F, de la Peña A, Llanquinao J, Vargas-Mardones G, Indo JA, Inestrosa NC, Tapia-Rojas C. Torres AK, et al. J Alzheimers Dis. 2021;84(4):1391-1414. doi: 10.3233/JAD-215139. J Alzheimers Dis. 2021. PMID: 34719499 Review. - Regional distribution of synaptic markers and APP correlate with distinct clinicopathological features in sporadic and familial Alzheimer's disease.
Shinohara M, Fujioka S, Murray ME, Wojtas A, Baker M, Rovelet-Lecrux A, Rademakers R, Das P, Parisi JE, Graff-Radford NR, Petersen RC, Dickson DW, Bu G. Shinohara M, et al. Brain. 2014 May;137(Pt 5):1533-49. doi: 10.1093/brain/awu046. Epub 2014 Mar 12. Brain. 2014. PMID: 24625695 Free PMC article. - Human tau increases amyloid β plaque size but not amyloid β-mediated synapse loss in a novel mouse model of Alzheimer's disease.
Jackson RJ, Rudinskiy N, Herrmann AG, Croft S, Kim JM, Petrova V, Ramos-Rodriguez JJ, Pitstick R, Wegmann S, Garcia-Alloza M, Carlson GA, Hyman BT, Spires-Jones TL. Jackson RJ, et al. Eur J Neurosci. 2016 Dec;44(12):3056-3066. doi: 10.1111/ejn.13442. Epub 2016 Nov 12. Eur J Neurosci. 2016. PMID: 27748574 Free PMC article. - Intraneuronal beta-amyloid accumulation and synapse pathology in Alzheimer's disease.
Gouras GK, Tampellini D, Takahashi RH, Capetillo-Zarate E. Gouras GK, et al. Acta Neuropathol. 2010 May;119(5):523-41. doi: 10.1007/s00401-010-0679-9. Epub 2010 Mar 31. Acta Neuropathol. 2010. PMID: 20354705 Free PMC article. Review.
Cited by
- Proteomic identification of select protein variants of the SNARE interactome associated with cognitive reserve in a large community sample.
Ramos-Miguel A, Jones AA, Petyuk VA, Barakauskas VE, Barr AM, Leurgans SE, De Jager PL, Casaletto KB, Schneider JA, Bennett DA, Honer WG. Ramos-Miguel A, et al. Acta Neuropathol. 2021 May;141(5):755-770. doi: 10.1007/s00401-021-02282-7. Epub 2021 Mar 1. Acta Neuropathol. 2021. PMID: 33646358 - Defective Transcytosis of APP and Lipoproteins in Human iPSC-Derived Neurons with Familial Alzheimer's Disease Mutations.
Woodruff G, Reyna SM, Dunlap M, Van Der Kant R, Callender JA, Young JE, Roberts EA, Goldstein LS. Woodruff G, et al. Cell Rep. 2016 Oct 11;17(3):759-773. doi: 10.1016/j.celrep.2016.09.034. Cell Rep. 2016. PMID: 27732852 Free PMC article. - Exosomal noncoding RNAs in central nervous system diseases: biological functions and potential clinical applications.
Wang ZY, Wen ZJ, Xu HM, Zhang Y, Zhang YF. Wang ZY, et al. Front Mol Neurosci. 2022 Nov 9;15:1004221. doi: 10.3389/fnmol.2022.1004221. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36438184 Free PMC article. Review. - The Interplay of Protein Aggregation, Genetics, and Oxidative Stress in Alzheimer's Disease: Role for Natural Antioxidants and Immunotherapeutics.
Ali J, Choe K, Park JS, Park HY, Kang H, Park TJ, Kim MO. Ali J, et al. Antioxidants (Basel). 2024 Jul 18;13(7):862. doi: 10.3390/antiox13070862. Antioxidants (Basel). 2024. PMID: 39061930 Free PMC article. Review. - Slow Wave Sleep Is a Promising Intervention Target for Alzheimer's Disease.
Lee YF, Gerashchenko D, Timofeev I, Bacskai BJ, Kastanenka KV. Lee YF, et al. Front Neurosci. 2020 Jun 30;14:705. doi: 10.3389/fnins.2020.00705. eCollection 2020. Front Neurosci. 2020. PMID: 32714142 Free PMC article. Review.
References
- Alzheimer A. Uber eine eigenartige Erkrankung der Hirnrinde. Allgemeine Zeitschrife Psychiatrie. 1907;64:146–148.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical