Reduced risk of hypoglycemia with once-daily glargine versus twice-daily NPH and number needed to harm with NPH to demonstrate the risk of one additional hypoglycemic event in type 2 diabetes: Evidence from a long-term controlled trial - PubMed (original) (raw)
Randomized Controlled Trial
Reduced risk of hypoglycemia with once-daily glargine versus twice-daily NPH and number needed to harm with NPH to demonstrate the risk of one additional hypoglycemic event in type 2 diabetes: Evidence from a long-term controlled trial
Julio Rosenstock et al. J Diabetes Complications. 2014 Sep-Oct.
Abstract
Aims: This analysis evaluated HbA1c-adjusted hypoglycemia risk with glargine versus neutral protamine Hagedorn (NPH) over a 5-year study in patients with Type 2 diabetes mellitus (T2DM). Clinical significance was assessed using number needed to harm (NNH) to demonstrate the risk of one additional patient experiencing at least one hypoglycemic event.
Methods: Individual patient-level data for symptomatic documented hypoglycemia and HbA1c values from a 5-year randomized study comparing once-daily glargine (n=513) with twice-daily NPH (n=504) were analyzed. Symptomatic hypoglycemia was categorized according to concurrent self-monitoring blood glucose levels and need for assistance. Hypoglycemic events per patient-year as a function of HbA1c were fitted by negative binomial regression using treatment and HbA1c at endpoint as independent variables. An estimate of NNH was derived from logistic regression models.
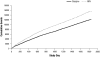
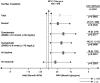
Results: The cumulative number of symptomatic hypoglycemia events was consistently lower with glargine compared with NPH over 5years. Compared with twice-daily NPH, once-daily glargine treatment resulted in significantly lower adjusted odds ratios (OR) for all daytime hypoglycemia (OR 0.74; p=0.030) and any severe event (OR 0.64; p=0.035), representing a 26% and 36% reduction in the odds of daytime and severe hypoglycemia, respectively. Our model predicts that, if 25 patients were treated with NPH instead of glargine, then one additional patient would experience at least one severe hypoglycemic event.
Conclusions: This analysis of long-term insulin treatment confirms findings from short-term studies and demonstrates that glargine provides sustained, clinically meaningful reductions in risk of hypoglycemia compared with NPH in patients with T2DM.
Keywords: Clinical diabetes; Clinical science and care; Glargine; Hypoglycemia; NPH.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest: J Rosenstock has served on scientific advisory boards and received honorarium or consulting fees from Roche, Sanofi, Novo Nordisk, Eli Lilly and Company, MannKind, GlaxoSmithKline, Takeda, Daiichi Sankyo, Johnson & Johnson, Novartis and Boehringer Ingelheim, and Amylin Pharmaceuticals. He has also received grants/research support from Merck, Pfizer, Sanofi, Novo Nordisk, Roche, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Takeda, Novartis, AstraZeneca, Amylin Pharmaceuticals, Johnson & Johnson, Daiichi Sankyo, MannKind, Intarcia Therapeutics and Boehringer Ingelheim. V Fonseca has received research support (to Tulane University) with grants from Novo Nordisk, Sanofi, Eli Lilly and Company, Daiichi Sankyo, Pamlabs, Reata Pharmaceuticals and Halozyme, and honoraria for consulting and lectures from GlaxoSmithKline, Takeda, Sanofi, Novo Nordisk, Daiichi Sankyo, Pamlabs, Xoma, AstraZeneca and Eli Lilly and Company. S Schinzel and MP Dain are employees of Sanofi. P Mullins has received grants and/or honoraria for consulting from Astra Zeneca, Boehringer Ingelheim, Merck, Novartis, Roche and Sanofi. M Riddle has received grants for research and/or honoraria for consulting or lectures from Amylin Pharmaceuticals, Eli Lilly and Company, the Amylin Lilly Alliance, Novo Nordisk, Pfizer, Sanofi and Valeritas; this conflict of interest has been reviewed and managed by Oregon Health & Science University.
Figures
Fig. 1
Cumulative number of symptomatic hypoglycemic events.
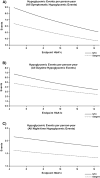
Fig. 2
Hypoglycemic events per person-year. (A) All symptomatic events; (B) all daytime events; (C) all nocturnal events. In all three regression analyses, the coefficient for endpoint HbA1c was not significantly different from zero. HbA1c = glycosylated hemoglobin; NPH = neutral protamne Hagedorn.
Fig. 3
HbA1c-adjusted number needed to harm analysis. *Defined as symptomatic hypoglycemia requiring assistance and having either SMBG ≤3.1 mmol/L or prompt recovery after oral carbohydrate, intravenous glucose, or glucagon administration; HbA1c = glycosylated hemoglobin; NPH = neutral protamine Hagedorn; NNH = number needed to harm; SMBG = self-monitoring of blood glucose; CI = confidence interval.
Similar articles
- (Ultra-)long-acting insulin analogues versus NPH insulin (human isophane insulin) for adults with type 2 diabetes mellitus.
Semlitsch T, Engler J, Siebenhofer A, Jeitler K, Berghold A, Horvath K. Semlitsch T, et al. Cochrane Database Syst Rev. 2020 Nov 9;11(11):CD005613. doi: 10.1002/14651858.CD005613.pub4. Cochrane Database Syst Rev. 2020. PMID: 33166419 Free PMC article. - A randomized trial comparing the rate of hypoglycemia--assessed using continuous glucose monitoring--in 125 preschool children with type 1 diabetes treated with insulin glargine or NPH insulin (the PRESCHOOL study).
Danne T, Philotheou A, Goldman D, Guo X, Ping L, Cali A, Johnston P. Danne T, et al. Pediatr Diabetes. 2013 Dec;14(8):593-601. doi: 10.1111/pedi.12051. Epub 2013 Jun 3. Pediatr Diabetes. 2013. PMID: 23730996 Clinical Trial. - Comparison of safety and efficacy of insulin glargine and neutral protamine hagedorn insulin in older adults with type 2 diabetes mellitus: results from a pooled analysis.
Lee P, Chang A, Blaum C, Vlajnic A, Gao L, Halter J. Lee P, et al. J Am Geriatr Soc. 2012 Jan;60(1):51-9. doi: 10.1111/j.1532-5415.2011.03773.x. J Am Geriatr Soc. 2012. PMID: 22239291 Clinical Trial. - Systematic review and meta-analysis of randomized clinical trials comparing efficacy and safety outcomes of insulin glargine with NPH insulin, premixed insulin preparations or with insulin detemir in type 2 diabetes mellitus.
Rys P, Wojciechowski P, Rogoz-Sitek A, Niesyczyński G, Lis J, Syta A, Malecki MT. Rys P, et al. Acta Diabetol. 2015 Aug;52(4):649-62. doi: 10.1007/s00592-014-0698-4. Epub 2015 Jan 14. Acta Diabetol. 2015. PMID: 25585592 Free PMC article. Review. - Systematic review and economic evaluation of a long-acting insulin analogue, insulin glargine.
Warren E, Weatherley-Jones E, Chilcott J, Beverley C. Warren E, et al. Health Technol Assess. 2004 Nov;8(45):iii, 1-57. doi: 10.3310/hta8450. Health Technol Assess. 2004. PMID: 15525480 Review.
Cited by
- Efficacy and safety of basal insulins in people with type 2 diabetes mellitus: a systematic review and network meta-analysis of randomized clinical trials.
Dehghani M, Sadeghi M, Barzkar F, Maghsoomi Z, Janani L, Motevalian SA, Loke YK, Ismail-Beigi F, Baradaran HR, Khamseh ME. Dehghani M, et al. Front Endocrinol (Lausanne). 2024 Mar 21;15:1286827. doi: 10.3389/fendo.2024.1286827. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38586456 Free PMC article. - Effect of Drugs Used in Pharmacotherapy of Type 2 Diabetes on Bone Density and Risk of Bone Fractures.
Wikarek A, Grabarczyk M, Klimek K, Janoska-Gawrońska A, Suchodolska M, Holecki M. Wikarek A, et al. Medicina (Kaunas). 2024 Feb 26;60(3):393. doi: 10.3390/medicina60030393. Medicina (Kaunas). 2024. PMID: 38541119 Free PMC article. Review. - Hepatic Insulin Resistance Model in the Male Wistar Rat Using Exogenous Insulin Glargine Administration.
Sarmiento-Ortega VE, Moroni-González D, Diaz A, García-González MÁ, Brambila E, Treviño S. Sarmiento-Ortega VE, et al. Metabolites. 2023 Apr 18;13(4):572. doi: 10.3390/metabo13040572. Metabolites. 2023. PMID: 37110230 Free PMC article. - (Ultra-)long-acting insulin analogues versus NPH insulin (human isophane insulin) for adults with type 2 diabetes mellitus.
Semlitsch T, Engler J, Siebenhofer A, Jeitler K, Berghold A, Horvath K. Semlitsch T, et al. Cochrane Database Syst Rev. 2020 Nov 9;11(11):CD005613. doi: 10.1002/14651858.CD005613.pub4. Cochrane Database Syst Rev. 2020. PMID: 33166419 Free PMC article. - Cost-Effectiveness of Insulin Glargine and Insulin Detemir in the Basal Regimen for Naïve Insulin Patients with Type 2 Diabetes Mellitus (T2DM) in Malaysia.
Shafie AA, Ng CH. Shafie AA, et al. Clinicoecon Outcomes Res. 2020 Jun 22;12:333-343. doi: 10.2147/CEOR.S244884. eCollection 2020. Clinicoecon Outcomes Res. 2020. PMID: 32606850 Free PMC article.
References
- Ali M, White J, Lee CH, Palmer JL, Smith-Palmer J, Fakhoury W, et al. Therapy conversion to biphasic insulin aspart 30 improves long-term outcomes and reduces the costs of type 2 diabetes in Saudi Arabia. Journal of Medical Economics. 2008;11:651–670. - PubMed
- Allicar MP, Megas F, Houzard S, Baroux A, Le Thai F, Augendre-Ferrante B. Frequency and costs of hospital stays for hypoglycemia in France in 1995. Presse Médicale. 2000;29:657–661. - PubMed
- Bazzano LA, Lee LJ, Shi L, Reynolds K, Jackson JA, Fonseca V. Safety and efficacy of glargine compared with NPH insulin for the treatment of type 2 diabetes: A meta-analysis of randomized controlled trials. Diabetic Medicine. 2008;25:924–932. - PubMed
- Bullano MF, Al-Zakwani IS, Fisher MD, Menditto L, Willey VJ. Differences in hypoglycemia event rates and associated cost-consequence in patients initiated on long-acting and intermediate-acting insulin products. Current Medical Research and Opinion. 2005;21:291–298. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical