Recovery of neuronal and network excitability after spinal cord injury and implications for spasticity - PubMed (original) (raw)
Review
Recovery of neuronal and network excitability after spinal cord injury and implications for spasticity
Jessica M D'Amico et al. Front Integr Neurosci. 2014.
Erratum in
- Front Integr Neurosci. 2014;8:49
Abstract
The state of areflexia and muscle weakness that immediately follows a spinal cord injury (SCI) is gradually replaced by the recovery of neuronal and network excitability, leading to both improvements in residual motor function and the development of spasticity. In this review we summarize recent animal and human studies that describe how motoneurons and their activation by sensory pathways become hyperexcitable to compensate for the reduction of functional activation of the spinal cord and the eventual impact on the muscle. Specifically, decreases in the inhibitory control of sensory transmission and increases in intrinsic motoneuron excitability are described. We present the idea that replacing lost patterned activation of the spinal cord by activating synaptic inputs via assisted movements, pharmacology or electrical stimulation may help to recover lost spinal inhibition. This may lead to a reduction of uncontrolled activation of the spinal cord and thus, improve its controlled activation by synaptic inputs to ultimately normalize circuit function. Increasing the excitation of the spinal cord with spared descending and/or peripheral inputs by facilitating movement, instead of suppressing it pharmacologically, may provide the best avenue to improve residual motor function and manage spasticity after SCI.
Keywords: motoneuron; noradrenaline; persistent inward currents; reflexes; serotonin; spinal cord injuries.
Figures
Figure 1
Re-emergence of PICs after chronic SCI. (A) Re-emergence of PICs in a completely transected (S2) rat after chronic (>50 days post-injury) SCI. Motoneuron recording in response to single pulse (3 × 's sensory threshold) dorsal root stimulation. At resting membrane potential (−65 mV, top trace), PIC activation produces a plateau potential and self-sustained firing. Hyperpolarization of the motoneuron (−75 mV, bottom trace) deactivates the PIC, eliminating the long-lasting reflex (LLR or spasm) and reveals the long (1 s) EPSP. (B) Spinal shock in acutely-spinalized rat (hours after injury), same preparation and experimental set-up as in (A). Note absence of long-lasting reflex response at resting membrane potential (−65 mV, top trace) due to elimination of PICs acutely after injury. The long-duration motoneuron EPSP (~1 s) appears acutely after injury as revealed in hyperpolarized motoneuron (−78 mV, bottom trace). Taken from Li et al. (2004a).
Figure 2
Slow, steady firing in rat and human motoneurons after chronic SCI. (A) Top trace: Firing response of a motoneuron from a chronic spinal rat in response to a brief depolarizing current pulse (~2 nA). Note low mean rate (1.75 Hz) and small standard deviation (±0.27 Hz) in firing. Bottom trace: Magnification of top trace indicated by rectangle. Plateau activation due to NaPIC is indicated by dotted line, and baseline membrane potential (Vm) is represented by solid black line. Note the ramp and acceleration (arrows) in Vm after each AHP due to NaPIC, producing interspike intervals much longer than the duration of the AHP (gray bar). (B) Top trace: Firing response of motoneuron from an acute spinal rat without plateau potentials in response to large (~4–5 nA) depolarizing current injections. Note increased firing rate (7.68 Hz) during large current pulse. Bottom trace: Magnification of top trace as indicated by rectangle. Interspike intervals are now closer to the duration of the AHP (gray bar) and lack NaPIC-mediated ramp in Vm. (A,B) taken from Li et al. (2004a). (C) Slow, steady firing rate of spontaneously-active soleus (SOL) and hamstrings (HAM) motor units from two participants with incomplete SCI (5 Hz firing). Note increase in firing rate and variability during superimposed voluntary activation of the muscles (10 Hz firing). (D) Relationship between the firing rate variability [standard deviation (SD)] and mean rate of motor units from SCI participants (black symbols) and control, uninjured participants (white symbols). (C,D) taken from Gorassini et al. (2004).
Figure 3
Estimating PICs in rat and human motoneurons. (A) Rat motoneurons: Paired motor unit analysis (ΔF) technique in rat motoneurons. (i,ii) Firing rate profiles of a control and test motoneuron in response to the same triangular current injection. Measurement of PIC as the difference in current input at recruitment and derecruitment of the test motoneuron (dashed lines): ΔI value. (iii) Linear relationship between the firing rate and injected current in the control motoneuron. (iv) The control motoneuron is serving as a measure of input to the test motoneuron. Measurement of the PIC as the difference in firing rate of the control motoneuron at recruitment and derecruitment (dashed lines) of the test motoneuron: ΔF value. (B) Human motoneurons: ΔF during muscle spasm in human SCI. Top trace: Tibialis anterior (TA) surface EMG during sustained dorsiflexion and application of transient vibration to TA tendon (gray squares) and medial arch of the foot (black square). Middle trace: Firing rate of a higher-threshold TA test unit in response to the vibrations. Bottom trace: Firing rate profile of tonically active lower-threshold control motor unit. ΔF is measured as the difference in control unit rate at derecruitment and recruitment of test motor unit (vertical lines and arrow). (A,B) taken from Gorassini et al. (2004).
Figure 4
Endogenous activation of 5HT2/NAα1 receptors with little monoamines below injury. (A) Top trace: Intracellular recording from motoneuron of chronic spinal rat. Single-pulse, dorsal root stimulation evokes long-lasting plateau potential and self-sustained firing. Bottom trace: Blocking of 5-HT2 and NAα1 receptors with cyproheptadine eliminates PIC-mediated plateau potential and self-sustained firing, leaving only sensory-mediated activation of motoneuron. (B) Immunofluorescence imaging of residual serotonergic fibers (beaded red) labeled with Texas-Red in spinal cords above (left) and below (right) a complete spinal transection from a rat spinalized 2 months previously. (B) was taken from Murray et al. (2010).
Figure 5
Mechanism of inverse agonists and neutral antagonists. Receptor state equilibrium: Inactive state (R, left panel) of G-protein coupled receptor (GPCR) where α, β, and γ subunits of the G-protein and downstream pathways are not activated. Active receptor state (R*, right panel) where the α-subunit is released by facilitating exchange of GDP for GTP. Dissociated α-subunit activates phospholipase C (PLC, not shown) resulting in the hydrolysis of phosphatidylinositol bisphosphate into the secondary messengers inositol trisphosphate (IP3) and diacylglycerol (DAG). IP3 increases calcium mobilization via release of intracellular, IP3-regulated calcium stores and DAG activates the downstream protein kinase C (PKC) which subsequently phosphorylates and activates voltage-gated channels such as the Na and Ca channels mediating the PICs.
Figure 6
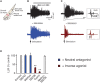
Constitutive receptor activity in chronic SCI rat. (A) In vitro ventral root recordings in response to single-pulse (3 × T) dorsal root stimulation in chronically spinalized (S2) rats. (B) PIC-mediated long-lasting reflex (LLR: 500–4000 ms after stimulation) before (black trace) and after (blue trace) application of the neutral antagonist SB242084. (C) Evoked LLR before (black trace) and after (red trace) application of the inverse agonist SB206553. Inset shows the sensory evoked, short-latency reflex (SLR: 10–40 ms) that is not mediated by the slow activating PICs or affected by the inverse agonist. (D) Group means of the LLR (expressed as a % of pre-drug values) after application of the neutral antagonists (blue), inverse agonists (red), and application of inverse agonist after the receptor is first blocked with the neutral antagonist (white) to render the inverse agonist ineffective. Taken from Murray et al. (2010). **p < 0.01.
Figure 7
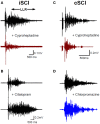
Constitutive receptor activity in chronic human SCI. (A) Inverse agonist cyproheptadine in a motor incomplete SCI participant (iSCI, AIS C). Unrectified surface EMG during long-lasting reflex (LLR) evoked in the TA muscle from medial arch stimulation before (top trace) and after (bottom trace) 8 mg of oral cyproheptadine. Note reduction in the presumably PIC-mediated long-lasting reflex (LLR: 500 ms after stimulation onwards). (B) Serotonin re-uptake inhibitor citalopram in iSCI (AIS C) participant. Long-lasting reflex (LLR) recorded in TA muscle before (top trace) and after (bottom trace) 20 mg of oral citalopram. (C) Same as in (A) but for 12 mg oral dose of cyproheptadine in motor and sensory complete SCI participant (cSCI, AIS A). (D) Effect of neutral antagonist chlorpromazine (12.5 mg) on PIC-mediated LLR in same cSCI participant. (A–C) taken from D'Amico et al. (2013b).
Figure 8
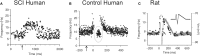
Peristimulus frequencygrams (PSF) in SCI and uninjured participants and in rat. (A) PSF of TA motor units recorded in incomplete SCI participant (AIS C) in response to stimulation of the medial arch of the foot (time marked by arrow, 0.2 ms pulse width, 3 pulses, 300 Hz, ~50 mA) while participant maintained tonic dorsiflexion. Mean rate indicated by solid gray line. (B) PSF recorded from uninjured control participant in response to same medial arch stimulation. Note shorter duration of response, pause and cluster of motor unit action potentials as marked by the dashed gray circle. (C) PSF (black dots) recorded in rat motoneuron that mimics the PSF obtained in the uninjured control participant from (B). The intracellular current injection profile required to obtain this PSF is shown in inset and the resulting Vm response (gray trace) to this injected profile in a hyperpolarized motoneuron. Taken from Norton et al. (2008).
Figure 9
Pre-synaptic and reciprocal inhibition in humans. (A) Pre-synaptic and reciprocal inhibitory pathways. Red trace: Monosynaptic Ia pathway to soleus (Sol, lower pathway) and homonymous activation of PAD interneurons (upper pathway). PAD interneurons marked by gray circles. Blue trace: Heteronymous, monosynaptic facilitation of soleus motoneurons by quadriceps (Q) Ia afferents that are tonically inhibited by PAD Ins, the latter activated by descending pathways (dashed line). Green trace: Reciprocal inhibitory pathway from tibialis anterior (TA) afferents onto soleus Ia reciprocal inhibitory interneuron (black circle) and onto soleus PAD interneuron via second order interneuron (D1/D2 inhibition). (B) PSTH obtained from a SCI participant in response to low-threshold electrical stimulation of the posterior tibial nerve (bottom red pathway in A). Note second peak in PSTH (arrow). (C) Decrease of second PSTH peak in response to paired activation (20 ms ISI) of posterior tibial nerve in uninjured control (top trace) but not SCI (bottom trace) participant indicative of reduced pre-synaptic inhibition (top red pathway in A). (B,C) were modified from Mailis and Ashby (1990).
Figure 10
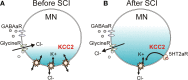
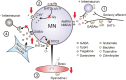
KCC2 cotransporter and chloride equilibrium before and after SCI. (A) A potassium chloride cotransporter (KCC2) removes chloride (Cl−) from the motoneuron (MN) in exchange for potassium (K+) to maintain Cl− equilibrium potential below resting membrane potential, allowing Cl− influx and MN hyperpolarization during activation of GABA and Glycine receptors (R). (B) Downregulation of KCC2 expression in motoneurons after SCI increases intracellular Cl− concentration, depolarizing Cl− equilibrium potential to above rest. This produces efflux of Cl− and depolarization of MN during activation of GABA and Glycine receptors. Activation of 5-HT2A receptors increases cell membrane expression of KCC2 after SCI to restore endogenous inhibition.
Figure 11
Effect of zolmitriptan on sensory transmission in SCI rat and human. (A) Effects of zolmitriptan in chronically injured rat motoneurons. (i)Top trace: Intracellular recording from chronic spinal rat motoneuron at rest (−72 mV) where single-pulse dorsal root stimulation produces PIC-mediated long-lasting reflex (LLR). Bottom trace: same motoneuron hyperpolarized to −80 mV to reveal short and long EPSP in response to dorsal root stimulation. (ii) Recording from same motoneuron in (i) at rest (top trace) and during hyperpolarization (bottom trace) after application of the 5HT1B/D receptor agonist zolmitriptan. Note elimination of the long-duration EPSP which prevents the triggering of the PIC-mediated long-lasting reflex. Taken from Murray et al. (2011b). (B) Unrectified EMG showing short- lasting reflex (SLR: first 50 ms of reflex response) recorded from the TA muscle in a single SCI participantbefore (top trace) and after (bottom trace) 10 mg oral zolmitriptan in response to medial arch stimulation. *Marks time of stimulation. (C) Unrectified TA EMG before (top trace) and after (bottom trace) 10 mg zolmitriptan in a single SCI participant in response to medial arch stimulation. Black bar denotes long-lasting reflex response (LLR: 500 ms after stimulation and beyond). (B,C) were taken from D'Amico et al. (2013a).
Figure 12
Target sites for current and future antispastic treatments. Pre-synaptic (1), motoneuron (2), muscle (3), and novel (4) sites for antispastic treatments. Site 1: GABAB, NAα2, and 5-HT1 receptors (r) located on pre-synaptic terminals or on interposed excitatory interneurons can be activated by baclofen, tizanidine, and zolmitriptan, respectively, to reduce excitatory glutamatergic release and activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and _N_-methyl-D-aspartate (NMDA) receptors located on the motoneurons. Site 2: 5-HT2 and NAα1 receptors on the motoneuron with constitutive or ligand activation facilitate downstream voltage-gated calcium channels (CaV) mediating PICs via Gq protein-coupled pathways. Inverse agonists, such as cyproheptadine, switch the 5HT2 and NAα1 receptors to their inactive state, reducing activity in the Gq pathway and lessening activation of PICs and consequently muscle spasms. Site 3: Botulinum toxin (Botox®) injection directly into spastic muscles or activation of ryanodine receptors by dantrolene sodium (dantrolene) reduces spasticity by targeting the muscle site directly. Site 4: Spinal injection of the HIV1-CMV-GAD65 lentivirus increases GAD65 gene expression and GABA release from astrocytes. Systemic administration of tiagabine, a GABA reuptake inhibitor, increases amount of enhanced GABA in synapse to activate pre- and post-synaptic GABA receptors to reduce motoneuron excitability. Activation of 5-HT2A receptors increases cell expression of KCC2 cotransporter to reduce intracellular concentration of Cl− and increase endogenous motoneuron inhibition. Adapted from D'Amico et al. (2013a).
Similar articles
- Muscle Spasms after Spinal Cord Injury Stem from Changes in Motoneuron Excitability and Synaptic Inhibition, Not Synaptic Excitation.
Mahrous A, Birch D, Heckman CJ, Tysseling V. Mahrous A, et al. J Neurosci. 2024 Jan 3;44(1):e1695232023. doi: 10.1523/JNEUROSCI.1695-23.2023. J Neurosci. 2024. PMID: 37949656 Free PMC article. - Persistent inward currents in spinal motoneurons: important for normal function but potentially harmful after spinal cord injury and in amyotrophic lateral sclerosis.
ElBasiouny SM, Schuster JE, Heckman CJ. ElBasiouny SM, et al. Clin Neurophysiol. 2010 Oct;121(10):1669-79. doi: 10.1016/j.clinph.2009.12.041. Epub 2010 May 11. Clin Neurophysiol. 2010. PMID: 20462789 Free PMC article. Review. - Reduction of spinal sensory transmission by facilitation of 5-HT1B/D receptors in noninjured and spinal cord-injured humans.
D'Amico JM, Li Y, Bennett DJ, Gorassini MA. D'Amico JM, et al. J Neurophysiol. 2013 Mar;109(6):1485-93. doi: 10.1152/jn.00822.2012. Epub 2012 Dec 5. J Neurophysiol. 2013. PMID: 23221401 Free PMC article. Clinical Trial. - Spinal inhibition and motor function in adults with spastic cerebral palsy.
Condliffe EG, Jeffery DT, Emery DJ, Gorassini MA. Condliffe EG, et al. J Physiol. 2016 May 15;594(10):2691-705. doi: 10.1113/JP271886. Epub 2016 Mar 17. J Physiol. 2016. PMID: 26842905 Free PMC article. - Human spinal cord injury: motor unit properties and behaviour.
Thomas CK, Bakels R, Klein CS, Zijdewind I. Thomas CK, et al. Acta Physiol (Oxf). 2014 Jan;210(1):5-19. doi: 10.1111/apha.12153. Epub 2013 Sep 13. Acta Physiol (Oxf). 2014. PMID: 23901835 Review.
Cited by
- Acute suppression of lower limb spasm by sacral afferent stimulation for people with spinal cord injury: A pilot study.
Massey S, Doherty S, Duffell L, Craggs M, Knight S. Massey S, et al. Wearable Technol. 2024 Apr 5;5:e9. doi: 10.1017/wtc.2024.4. eCollection 2024. Wearable Technol. 2024. PMID: 38617468 Free PMC article. - Distinct patterns of spasticity and corticospinal connectivity following complete spinal cord injury.
Sangari S, Kirshblum S, Guest JD, Oudega M, Perez MA. Sangari S, et al. J Physiol. 2021 Oct;599(19):4441-4454. doi: 10.1113/JP281862. Epub 2021 Sep 16. J Physiol. 2021. PMID: 34107068 Free PMC article. - Prevalence of spasticity in humans with spinal cord injury with different injury severity.
Sangari S, Perez MA. Sangari S, et al. J Neurophysiol. 2022 Sep 1;128(3):470-479. doi: 10.1152/jn.00126.2022. Epub 2022 May 4. J Neurophysiol. 2022. PMID: 35507475 Free PMC article. - The functional properties of synapses made by regenerated axons across spinal cord lesion sites in lamprey.
Parker D. Parker D. Neural Regen Res. 2022 Oct;17(10):2272-2277. doi: 10.4103/1673-5374.335828. Neural Regen Res. 2022. PMID: 35259849 Free PMC article. - Effects of non-invasive cervical spinal cord neuromodulation by trans-spinal electrical stimulation on cortico-muscular descending patterns in upper extremity of chronic stroke.
Zhang J, Wang M, Alam M, Zheng YP, Ye F, Hu X. Zhang J, et al. Front Bioeng Biotechnol. 2024 Mar 21;12:1372158. doi: 10.3389/fbioe.2024.1372158. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38576448 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources