Mitochondrial oxidative stress in aging and healthspan - PubMed (original) (raw)
Review
Mitochondrial oxidative stress in aging and healthspan
Dao-Fu Dai et al. Longev Healthspan. 2014.
Abstract
The free radical theory of aging proposes that reactive oxygen species (ROS)-induced accumulation of damage to cellular macromolecules is a primary driving force of aging and a major determinant of lifespan. Although this theory is one of the most popular explanations for the cause of aging, several experimental rodent models of antioxidant manipulation have failed to affect lifespan. Moreover, antioxidant supplementation clinical trials have been largely disappointing. The mitochondrial theory of aging specifies more particularly that mitochondria are both the primary sources of ROS and the primary targets of ROS damage. In addition to effects on lifespan and aging, mitochondrial ROS have been shown to play a central role in healthspan of many vital organ systems. In this article we review the evidence supporting the role of mitochondrial oxidative stress, mitochondrial damage and dysfunction in aging and healthspan, including cardiac aging, age-dependent cardiovascular diseases, skeletal muscle aging, neurodegenerative diseases, insulin resistance and diabetes as well as age-related cancers. The crosstalk of mitochondrial ROS, redox, and other cellular signaling is briefly presented. Potential therapeutic strategies to improve mitochondrial function in aging and healthspan are reviewed, with a focus on mitochondrial protective drugs, such as the mitochondrial antioxidants MitoQ, SkQ1, and the mitochondrial protective peptide SS-31.
Keywords: Aging; Healthspan; Mitochondria; Oxidative stress.
Figures
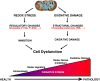
Figure 1
Illustration of the continuum of oxidative stress in health and pathology. The redox stress pathway emphasizes the signaling role of oxidative stress and focuses on reversible regulation and depends on the interaction between cellular components and the redox environment of the cell. In contrast, prolonged or high oxidative stress leads to structural changes in proteins, lipids, and DNA that are generally more irreversible. These represent two points along the continuum of how oxidative stress may contribute to aging phenotypes. Modified from Marcinek and Siegel [120].
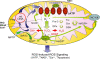
Figure 2
Interdependences of mtROS, nicotinamide nucleotides, and SIRT3: ROS-Induced ROS Signaling. Modified from Dai et al. [93].
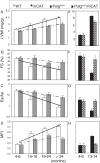
Figure 3
Echocardiography of cardiac aging in wild-type (WT) and mCAT mice (A-D) and Polg m/m mice in the presence or absence of mCAT (E-H). (A, E) Left ventricular mass index (LVMI), (B, F) % FS (fractional shortening), (C, G) Ea/Aa by tissue Doppler imaging (diastolic function), (D, H) the myocardial performance index (MPI). The increased linear trends across ages in WT mice were significant for all parameters (P <0.05 for all, left panels). The beneficial effect of mCAT versus WT was analyzed by the interaction between genotype and the linear age trend, and was significant in all cases (P <0.01 for all except fractional shortening, P = 0.03). *P <0.05 versus Polgm/m at age 4 to 6 months, #P <0.05 versus Polgm/m at age 13 to 14 months (right panels). LVMI, Left ventricular mass index; mCAT, catalase targeted to mitochondria. Modified from Dai et al. [25,43].
Figure 4
Mitochondrial oxidative damage and mtDNA deletions in cardiac aging. (A). Mitochondrial protein carbonyl (nmol/mg) significantly increased in old wild-type (OWT, >24 months) and even more in middle-aged Polg (13.5 months) mouse hearts when compared with young WT mouse hearts. mCAT significantly reduced the age-dependent mitochondrial protein carbonylation. (B) Mitochondrial DNA deletion frequency significantly increased in OWT (>24 months) and young Polg (4 months) when compared with young WT, and this is dramatically increased in middle-aged Polg (13.5 months). mCAT overexpression significantly reduced the deletion frequency for both. *P <0.05 compared with YWT. Modified from Dai et al. [25,43].
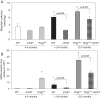
Figure 5
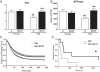
Mitochondrial targeted SS-31 improves skeletal muscle function.In vivo mitochondrial coupling ratio (P/O) (A) and maximum mitochondrial ATP production (B) in the hindlimb muscles of aged mice were both increased 1 h after treatment with SS-31. In situ fatigue resistance in the aged mice was also increased 1 h after SS-31 treatment (C). Eight days of daily treatment with SS-31 led to increased endurance capacity in the aged mice (D) as well. Means ± SEM. n = 5-7 per group. **P <0.01 relative to age-matched control. ##P <0.01 relative to young control. Young - 5 months old; Old - 27 months old. Modified from Siegel et al. [54].
Similar articles
- Oxidative Stress and the Aging Brain: From Theory to Prevention.
Gemma C, Vila J, Bachstetter A, Bickford PC. Gemma C, et al. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 15. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 15. PMID: 21204345 Free Books & Documents. Review. - Mitochondrial biogenesis: pharmacological approaches.
Valero T. Valero T. Curr Pharm Des. 2014;20(35):5507-9. doi: 10.2174/138161282035140911142118. Curr Pharm Des. 2014. PMID: 24606795 - Mitochondrial Oxidative Stress, Mitochondrial DNA Damage and Their Role in Age-Related Vascular Dysfunction.
Mikhed Y, Daiber A, Steven S. Mikhed Y, et al. Int J Mol Sci. 2015 Jul 13;16(7):15918-53. doi: 10.3390/ijms160715918. Int J Mol Sci. 2015. PMID: 26184181 Free PMC article. Review. - Mitochondrial-Targeted Catalase: Extended Longevity and the Roles in Various Disease Models.
Dai DF, Chiao YA, Martin GM, Marcinek DJ, Basisty N, Quarles EK, Rabinovitch PS. Dai DF, et al. Prog Mol Biol Transl Sci. 2017;146:203-241. doi: 10.1016/bs.pmbts.2016.12.015. Epub 2017 Feb 4. Prog Mol Biol Transl Sci. 2017. PMID: 28253986 Review. - Mitochondrial function and redox control in the aging eye: role of MsrA and other repair systems in cataract and macular degenerations.
Brennan LA, Kantorow M. Brennan LA, et al. Exp Eye Res. 2009 Feb;88(2):195-203. doi: 10.1016/j.exer.2008.05.018. Epub 2008 Jun 7. Exp Eye Res. 2009. PMID: 18588875 Free PMC article. Review.
Cited by
- Sarcopenia in Chronic Kidney Disease: Focus on Advanced Glycation End Products as Mediators and Markers of Oxidative Stress.
Dozio E, Vettoretti S, Lungarella G, Messa P, Corsi Romanelli MM. Dozio E, et al. Biomedicines. 2021 Apr 9;9(4):405. doi: 10.3390/biomedicines9040405. Biomedicines. 2021. PMID: 33918767 Free PMC article. Review. - Effects of 5-fluorouracil on morphology, cell cycle, proliferation, apoptosis, autophagy and ROS production in endothelial cells and cardiomyocytes.
Focaccetti C, Bruno A, Magnani E, Bartolini D, Principi E, Dallaglio K, Bucci EO, Finzi G, Sessa F, Noonan DM, Albini A. Focaccetti C, et al. PLoS One. 2015 Feb 11;10(2):e0115686. doi: 10.1371/journal.pone.0115686. eCollection 2015. PLoS One. 2015. PMID: 25671635 Free PMC article. - Risk factors associated with reproductive performance in small-scale dairy farms in Mexico.
Montiel-Olguín LJ, Estrada-Cortés E, Espinosa-Martínez MA, Mellado M, Hernández-Vélez JO, Martínez-Trejo G, Ruiz-López FJ, Vera-Avila HR. Montiel-Olguín LJ, et al. Trop Anim Health Prod. 2019 Jan;51(1):229-236. doi: 10.1007/s11250-018-1681-9. Epub 2018 Aug 9. Trop Anim Health Prod. 2019. PMID: 30094583 - Regulation of immune cell function by nicotinamide nucleotide transhydrogenase.
Regan T, Conway R, Bharath LP. Regan T, et al. Am J Physiol Cell Physiol. 2022 Apr 1;322(4):C666-C673. doi: 10.1152/ajpcell.00607.2020. Epub 2022 Feb 9. Am J Physiol Cell Physiol. 2022. PMID: 35138175 Free PMC article. Review. - Surface Lin28A expression consistent with cellular stress parallels indicators of senescence.
Broughton K, Esquer C, Echeagaray O, Firouzi F, Shain G, Ebeid D, Monsanto M, Yaareb D, Golgolab L, Gude N, Sussman MA. Broughton K, et al. Cardiovasc Res. 2023 May 2;119(3):743-758. doi: 10.1093/cvr/cvac122. Cardiovasc Res. 2023. PMID: 35880724 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources