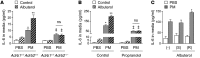β₂-Adrenergic agonists augment air pollution-induced IL-6 release and thrombosis - PubMed (original) (raw)
. 2014 Jul;124(7):2935-46.
doi: 10.1172/JCI75157. Epub 2014 May 27.
Saul Soberanes, Daniela Urich, Luisa Morales-Nebreda, Recep Nigdelioglu, David Green, James B Young, Angel Gonzalez, Carmen Rosario, Alexander V Misharin, Andrew J Ghio, Richard G Wunderink, Helen K Donnelly, Kathryn A Radigan, Harris Perlman, Navdeep S Chandel, G R Scott Budinger, Gökhan M Mutlu
- PMID: 24865431
- PMCID: PMC4071386
- DOI: 10.1172/JCI75157
β₂-Adrenergic agonists augment air pollution-induced IL-6 release and thrombosis
Sergio E Chiarella et al. J Clin Invest. 2014 Jul.
Abstract
Acute exposure to particulate matter (PM) air pollution causes thrombotic cardiovascular events, leading to increased mortality rates; however, the link between PM and cardiovascular dysfunction is not completely understood. We have previously shown that the release of IL-6 from alveolar macrophages is required for a prothrombotic state and acceleration of thrombosis following exposure to PM. Here, we determined that PM exposure results in the systemic release of catecholamines, which engage the β2-adrenergic receptor (β2AR) on murine alveolar macrophages and augment the release of IL-6. In mice, β2AR signaling promoted the development of a prothrombotic state that was sufficient to accelerate arterial thrombosis. In primary human alveolar macrophages, administration of a β2AR agonist augmented IL-6 release, while the addition of a beta blocker inhibited PM-induced IL-6 release. Genetic loss or pharmacologic inhibition of the β2AR on murine alveolar macrophages attenuated PM-induced IL-6 release and prothrombotic state. Furthermore, exogenous β2AR agonist therapy further augmented these responses in alveolar macrophages through generation of mitochondrial ROS and subsequent increase of adenylyl cyclase activity. Together, these results link the activation of the sympathetic nervous system by β2AR signaling with metabolism, lung inflammation, and an enhanced susceptibility to thrombotic cardiovascular events.
Figures
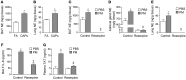
Figure 1. Catecholamines are required for PM-induced inflammation and thrombosis.
We exposed wild-type (C57BL/6) mice contemporaneously to either CAPs (PM2.5) or filtered air (FA) for 8 hours daily on 3 consecutive days and measured levels of norepinephrine (NE) in the (A) BAT and (B) lung tissue. We also performed intratracheal instillations of PM (200 μg/mouse) or control (PBS) in wild-type mice and gave reserpine (5 mg/kg 100 μl) or vehicle (20% ascorbic acid 100 μl) by gavage once 4 hours before treatment with PM or control (PBS). Twenty-four hours later, we measured levels of norepinephrine in the (C) BAT, (D) adrenal gland, and (E) lung. In identically treated mice, we measured (F) IL-6 in the BALF and (G) TAT complexes in the plasma. *P < 0.05, CAPs vs. FA, PM vs. PBS; ‡ P < 0.05, reserpine vs. control.
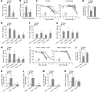
Figure 2. β2ARs are required for the catecholamine-induced upregulation of IL-6 and the resulting thrombosis after PM exposure.
We pretreated mice with propranolol (3 mg/kg in 100 μl i.p.) or vehicle (saline 100 μl i.p.) every 8 hours, followed 24 hours later by PM (200 μg/mouse) or control (PBS) and measured (A) the levels of IL-6 in the BALF, (B) the plasma levels of TAT complexes, and (C) the time to loss of blood flow after FeCl3-induced injury to the carotid artery. (D) The time to complete (>75% reduction) loss of blood flow is shown. We performed intratracheal instillations in mice lacking either β1AR (Adrb1–/–Adrb2+/+), β2AR (Adrb1+/+Adrb2–/–), or both β1AR and β2AR (Adrb1–/–Adrb2–/–) and their wild-type littermate controls (Adrb1+/+Adrb2+/+) with PM or PBS and, 24 hours later, measured the levels of (E) IL-6, (F) TNF-α, and (G) MCP-1 in BALF, (H) the plasma levels of TAT complexes in the plasma, and (I and J) the time to loss of blood flow after FeCl3-induced injury to the carotid artery. We exposed β2AR deficient mice (Adrb1+/+Adrb2–/–) and wild-type littermate controls (Adrb1+/+Adrb2+/+) contemporaneously to either CAPs (PM2.5) or filtered air for 8 hours daily on 3 consecutive days and measured levels of (K) Il6, (L) SP-B, and (M) TF mRNA in the lung tissue and (N) TAT complexes in the plasma. *P < 0.05, PM vs. PBS or CAPs vs. FA; ‡P < 0.05, propranolol vs. control_,_ Adrb1+/+Adrb2–/– and Adrb1–/–Adrb2–/– vs. Adrb1–/–Adrb2+/+ and Adrb1+/+Adrb2+/+.
Figure 3. In human alveolar macrophages, stimulation of β2AR augments and inhibition of βAR inhibits PM-induced IL-6 release.
We treated primary human alveolar macrophages with PM (10 μg/cm2) or control (medium) and with albuterol (10–7M) or control (medium) in the presence or absence of propranolol (10 μM) or control (saline) and measured IL-6 levels in the medium 24 hours later. *P < 0.05, PM vs. PBS; ** P < 0.05, albuterol vs. control; ‡P < 0.05, propranolol, vs. control.
Figure 4. β2ARs on alveolar macrophages are required for catecholamine-induced upregulation of IL-6 and resulting thrombosis after PM exposure.
(A and B) Mice deficient in β2AR specifically in monocytes and macrophages were generated by crossing Lysm-Cre mice with Adrb2flox/flox mice. Inflammatory cells from lung homogenates of Adrb2flox/flox and Lysm-Cre Adrb2flox/flox mice were sorted, and deletion of the Adrb2 gene was confirmed by assessment of (A) gene expression and (B) mRNA. We performed intratracheal instillations in Adrb2flox/flox and Lysm-Cre Adrb2flox/flox mice with PM (200 μg/mouse) or vehicle (PBS) and, 24 hours later, measured (C) the levels of IL-6 in BALF, (D) TAT complexes in the plasma, and (E and F) the time to loss of blood flow after FeCl3-induced injury to the carotid artery (the time to complete loss of blood flow [>75% reduction]). *P < 0.05, PM vs. PBS or CAPs vs. FA; ‡P < 0.05, Adrb2flox/flox vs. Lysm-Cre Adrb2flox/flox.
Figure 5. Treatment with β2 agonists worsens PM-induced release of IL-6 and resulting thrombosis.
We treated wild-type mice with formoterol (10–5M) or vehicle control (ethanol 2%), both with 1 ml via inhalation over 30 minutes every 12 hours, and exposed them contemporaneously to CAPs (PM2.5) or filtered air for 8 hours daily on 3 consecutive days. We measured levels of (A) IL-6 in BALF, (B) Il6 mRNA in lung tissue, (C) prothrombin (factor II), and (D) TF mRNA in liver and (E) the time to loss of blood flow after FeCl3-induced injury to the carotid artery (the time to complete loss of blood flow [>75% reduction]). *P < 0.05, PM vs. PBS or CAPs vs. FA; **P < 0.05, formoterol vs. control; ‡P < 0.05, Adrb2flox/flox vs. Lysm-Cre Adrb2flox/flox.
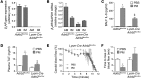
Figure 6. Engagement of β2AR augments PM-induced release of IL-6 from alveolar macrophages in vitro.
(A) We treated primary alveolar macrophages isolated from Adrb2–/– mice (Adrb1+/+Adrb2–/–) and their wild-type littermate controls (Adrb1+/+Adrb2+/+) with PM (10 μg/cm2) or control (medium) and measured IL-6 levels in the medium 24 hours later. (B) We treated murine alveolar macrophages (MH-S cells) with PM (10 μg/cm2) or control (medium) and with albuterol (10–7M) or control (medium) in the presence or absence of propranolol (10 μM) (or control [PBS]) and measured IL-6 levels in the medium 24 hours later. (C) We treated MH-S cells with PM (10 μg/cm2) or control (medium) and with the (S) or (R) enantiomer of albuterol (10–7M) or control. *P < 0.05 for comparison between PM-treated cells with or without albuterol or forskolin; *P < 0.05, PM vs. PBS; **P < 0.05, albuterol vs. control, and simultaneous vs. control; ‡P < 0.05, Adrb1+/+Adrb2–/– vs. Adrb1+/+Adrb2+/+, propranolol vs. control.
Figure 7. β2 Agonist therapy and forskolin augment PM-induced release of IL-6 only when administered following exposure to PM.
We treated MH-S cells with PM (10 μg/cm2) or control (medium) and with (A) albuterol (10–7M) or (B) forskolin (50 μM) and control administered simultaneously with the PM or 1 hour after the PM. (C) We treated MH-S cells with PM (10 μg/cm2) or control (medium) and with forskolin (50 μM) or control (saline) 1 hour after PM treatment in the absence of presence of an adenylyl cyclase (AC) inhibitor (SQ22536, 300 μM) (or control [H2O]) and measured IL-6 levels in the medium 24 hours later. (D) We treated MH-S cells with PM (10 μg/cm2) or control (medium) and with forskolin (50 μM) or control 1 hour after PM treatment in the absence or presence of IBMX (1 μM) and aminophylline (10 μM) and measured cAMP levels in cell lysates 1 minute later. *P < 0.05, PM vs. PBS; **P < 0.05, albuterol or forskolin (1 hour after PM) vs. control and albuterol or forskolin (simultaneous with PM ); ‡ P < 0.05, AC inhibitor vs. control.
Figure 8. PM-induced ROS generation and priming of adenylyl cyclase are required for β2 agonist–mediated worsening of IL-6 release.
We treated (A) MH-S cells and (B) primary alveolar macrophages (AM) with PM (10 μg/cm2) or control (medium) and measured IL-6 levels in the absence or presence of a mitochondrially targeted antioxidant (Mito-Q or control [TPP]) or (C) a nontargeted antioxidant (EUK-134) (20 μM). (D) We treated MH-S cells with PM or control and with forskolin (50 μM) or control and measured cAMP levels 1 minute after forskolin treatment. (E) We treated MH-S cells with antimycin A (AA) (1 μM) or vehicle and with forskolin (50 μM) and measured IL-6 in the medium 24 hours later in the absence or presence of stigmatellin (1 μM). (F) We treated MH-S cells with PM or control and with forskolin (50 μM) or control and measured IL-6 levels 24 hours later in the absence or presence of stigmatellin. (G) We treated MH-S cells with PM or control and with EUK-134 or control and performed immunoblotting against cAMP CREB in the nuclear and cytoplasmic fractions 4 hours later. (H) We measured IL-6 levels in control and CREB shRNA–transfected MH-S cells after treatment with PM or PBS. (I) We treated control and p65-shRNA–transfected cells with PM or control and with albuterol or control and measured IL-6 levels. *P < 0.05, PM vs. PBS; **P < 0.05, albuterol or forskolin vs. control; ‡P < 0.05, Adrb1+/+Adrb2–/– vs. Adrb1+/+Adrb2+/+, AC inhibitor, mito-Q, EUK-134, or stigmatellin vs. control, CREB or p65 vs. control shRNA.
Similar articles
- Synergistic Inhibition of β2-adrenergic Receptor-mediated Alveolar Epithelial Fluid Transport by Interleukin-8 and Transforming Growth Factor-β.
Wagener BM, Roux J, Carles M, Pittet JF. Wagener BM, et al. Anesthesiology. 2015 May;122(5):1084-92. doi: 10.1097/ALN.0000000000000595. Anesthesiology. 2015. PMID: 25591042 - Metformin Targets Mitochondrial Electron Transport to Reduce Air-Pollution-Induced Thrombosis.
Soberanes S, Misharin AV, Jairaman A, Morales-Nebreda L, McQuattie-Pimentel AC, Cho T, Hamanaka RB, Meliton AY, Reyfman PA, Walter JM, Chen CI, Chi M, Chiu S, Gonzalez-Gonzalez FJ, Antalek M, Abdala-Valencia H, Chiarella SE, Sun KA, Woods PS, Ghio AJ, Jain M, Perlman H, Ridge KM, Morimoto RI, Sznajder JI, Balch WE, Bhorade SM, Bharat A, Prakriya M, Chandel NS, Mutlu GM, Budinger GRS. Soberanes S, et al. Cell Metab. 2019 Feb 5;29(2):335-347.e5. doi: 10.1016/j.cmet.2018.09.019. Epub 2018 Oct 11. Cell Metab. 2019. PMID: 30318339 Free PMC article. - Upregulation of alveolar epithelial active Na+ transport is dependent on beta2-adrenergic receptor signaling.
Mutlu GM, Dumasius V, Burhop J, McShane PJ, Meng FJ, Welch L, Dumasius A, Mohebahmadi N, Thakuria G, Hardiman K, Matalon S, Hollenberg S, Factor P. Mutlu GM, et al. Circ Res. 2004 Apr 30;94(8):1091-100. doi: 10.1161/01.RES.0000125623.56442.20. Epub 2004 Mar 11. Circ Res. 2004. PMID: 15016730 - ROS-mediated regulation of β2AR function: Does oxidation play a meaningful role towards β2-agonist tachyphylaxis in airway obstructive diseases?
Teyani RL, Moghaddam F, Moniri NH. Teyani RL, et al. Biochem Pharmacol. 2024 Aug;226:116403. doi: 10.1016/j.bcp.2024.116403. Epub 2024 Jun 28. Biochem Pharmacol. 2024. PMID: 38945277 Review. - The β2-adrenergic receptor-ROS signaling axis: An overlooked component of β2AR function?
Rambacher KM, Moniri NH. Rambacher KM, et al. Biochem Pharmacol. 2020 Jan;171:113690. doi: 10.1016/j.bcp.2019.113690. Epub 2019 Nov 5. Biochem Pharmacol. 2020. PMID: 31697929 Free PMC article. Review.
Cited by
- β2-Adrenergic agonists attenuate organic dust-induced lung inflammation.
Romberger DJ, Heires AJ, Nordgren TM, Poole JA, Toews ML, West WW, Wyatt TA. Romberger DJ, et al. Am J Physiol Lung Cell Mol Physiol. 2016 Jul 1;311(1):L101-10. doi: 10.1152/ajplung.00125.2016. Epub 2016 May 17. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27190062 Free PMC article. - Air Pollution, Asthma, and Sleep Apnea: New Epidemiological Links?
Mutlu GM, Peker Y. Mutlu GM, et al. Ann Am Thorac Soc. 2019 Mar;16(3):307-308. doi: 10.1513/AnnalsATS.201812-924ED. Ann Am Thorac Soc. 2019. PMID: 31339334 Free PMC article. No abstract available. - Monocyte-Derived Macrophages Are Necessary for Beta-Adrenergic Receptor-Driven Choroidal Neovascularization Inhibition.
Droho S, Cuda CM, Perlman H, Lavine JA. Droho S, et al. Invest Ophthalmol Vis Sci. 2019 Dec 2;60(15):5059-5069. doi: 10.1167/iovs.19-28165. Invest Ophthalmol Vis Sci. 2019. PMID: 31800964 Free PMC article. - PM2.5 exposure associated with microbiota gut-brain axis: Multi-omics mechanistic implications from the BAPE study.
Li T, Fang J, Tang S, Du H, Zhao L, Wang Y, Deng F, Liu Y, Du Y, Cui L, Shi W, Wang Y, Wang J, Zhang Y, Dong X, Gao Y, Shen Y, Dong L, Zhou H, Sun Q, Dong H, Peng X, Zhang Y, Cao M, Zhi H, Zhou J, Shi X. Li T, et al. Innovation (Camb). 2022 Feb 3;3(2):100213. doi: 10.1016/j.xinn.2022.100213. eCollection 2022 Mar 29. Innovation (Camb). 2022. PMID: 35243467 Free PMC article. - Impaired clearance of influenza A virus in obese, leptin receptor deficient mice is independent of leptin signaling in the lung epithelium and macrophages.
Radigan KA, Morales-Nebreda L, Soberanes S, Nicholson T, Nigdelioglu R, Cho T, Chi M, Hamanaka RB, Misharin AV, Perlman H, Budinger GR, Mutlu GM. Radigan KA, et al. PLoS One. 2014 Sep 18;9(9):e108138. doi: 10.1371/journal.pone.0108138. eCollection 2014. PLoS One. 2014. PMID: 25232724 Free PMC article.
References
- UN Environment Program World Health Organization. Air Pollution In The World’s Megacities: A Report From The UN Environment Programme And Who Environment. New York, New York, USA: United Nations; 1994;35–37.
Publication types
MeSH terms
Substances
Grants and funding
- ES013995/ES/NIEHS NIH HHS/United States
- R01 ES013995/ES/NIEHS NIH HHS/United States
- HL071643/HL/NHLBI NIH HHS/United States
- I01 BX000201/BX/BLRD VA/United States
- UL1 RR025741/RR/NCRR NIH HHS/United States
- P01 HL071643/HL/NHLBI NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- R01 ES015024/ES/NIEHS NIH HHS/United States
- ES015024/ES/NIEHS NIH HHS/United States
- T32 HL076139/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases