A novel de novo mutation of SCN8A (Nav1.6) with enhanced channel activation in a child with epileptic encephalopathy - PubMed (original) (raw)
Case Reports
A novel de novo mutation of SCN8A (Nav1.6) with enhanced channel activation in a child with epileptic encephalopathy
Mark Estacion et al. Neurobiol Dis. 2014 Sep.
Abstract
Rare de novo mutations of sodium channels are thought to be an important cause of sporadic epilepsy. The well established role of de novo mutations of sodium channel SCN1A in Dravet Syndrome supports this view, but the etiology of many cases of epileptic encephalopathy remains unknown. We sought to identify the genetic cause in a patient with early onset epileptic encephalopathy by whole exome sequencing of genomic DNA. The heterozygous mutation c. 2003C>T in SCN8A, the gene encoding sodium channel Nav1.6, was detected in the patient but was not present in either parent. The resulting missense substitution, p.Thr767Ile, alters an evolutionarily conserved residue in the first transmembrane segment of channel domain II. The electrophysiological effects of this mutation were assessed in neuronal cells transfected with mutant or wildtype cDNA. The mutation causes enhanced channel activation, with a 10mV depolarizing shift in voltage dependence of activation as well as increased ramp current. In addition, pyramidal hippocampal neurons expressing the mutant channel exhibit increased spontaneous firing with PDS-like complexes as well as increased frequency of evoked action potentials. The identification of this new gain-of-function mutation of Nav1.6 supports the inclusion of SCN8A as a causative gene in infantile epilepsy, demonstrates a novel mechanism for hyperactivity of Nav1.6, and further expands the role of de novo mutations in severe epilepsy.
Keywords: De novo mutation; Epilepsy; Epileptic encephalopathy; Sodium channel.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
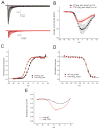
Figure 1. Location of the p.Thr767Ile mutation in Nav1.6 and evolutionary conservation of the mutated residue
(A) Threonine 767 is located in transmembrane segment 1 of domain II of the voltage-gated sodium channel alpha subunit. (B) This threonine residue is conserved in the vertebrate SCN8A channel from reptile (a, anotole) and fish (f, Fugu) and in the homologous invertebrate channel from drosophila and Ciona. (C) Threonine (T) at position 767 is conserved in the members of the human voltage-gated sodium channel gene family, including muscle and cardiac channels SCN4A and SCN5A, respectively.
Figure 2. Voltage-clamp analysis of Nav1.6 p.Thr767Ile mutant channels
(A) Representative traces recorded from ND7/23 cells expressing mNav1.6R (WT, black lines) and T767I (red lines) in response to the activation stimulation protocol (100 msec duration pulses). (B) The peak currents are normalized for cell capacitance and then are averaged together to obtain the activation current-voltage (I–V) relation for cells expressing WT (black symbols) or T767I (red symbols) as described in the Methods. (C) The G-V curves for both WT (black symbols) and T767I (red symbols) cell are normalized and then averaged to obtain the Boltzmann fit for activation data, which shows hyperpolarizing shift of activation of T767I channels compared to WT channels. (D) The responses to the fast-inactivation protocol are analyzed to obtain the voltage-dependence of fast-inactivation as described in the methods. The normalized and averaged and the Boltzmann’s fit are shown for both WT (black symbols) and T767I (red symbols). The voltage-dependence of fast-inactivation of T767I channels is not different from that of WT channels. Error bars are standard error of the mean (SEM). (E) The averaged currents evoked during a slow ramp stimulus beginning at −100 mV and ending at 20 mV over a duration of 600 msec are shown from WT (black line, n=6) and T767I (red line, n=10) expressing cells. The traces have been normalized to the maximal peak current recorded from that cell. T767I channels produce a larger normalized ramp current which peaks at a more hyperpolarized voltage compared to that of WT channels.
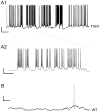
Figure 3. Neurons expressing Nav1.6 - T767I exhibit an increase in spontaneous activity
Continuous segments of current-clamp recording from hippocampal pyramidal neurons illustrate patterns of spontaneous action potential firing seen in a subset of cells expressing T767I channels. (A1) Trace from a representative spontaneously active cell. Scale bar indicates 20 mV vertical, 5 sec horizontal. (A2) A small region of the trace was expanded to illustrate firing behavior riding on top of transient depolarizations of the membrane potential, paroxsysmal depolarizing shift-like complexes, characteristic of epileptic discharges. Scale bar indicates 20mV vertical, 1sec horizontal. (B) Trace from a hippocampal pyramidal cell expressing Nav1.6-WT with the same scaling as panel A1 for comparison. Scale bar indicates 20 mV vertical, 5 sec horizontal.
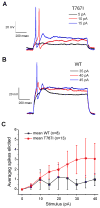
Figure 4. Neurons expressing Nav1.6 - T767I are hyperexcitable compared to those expressing the WT channel
Selected traces illustrating action potentials elicited near threshold stimulus current injections for Nav1.6-T767I expressing (A) and Nav1.6-WT expressing (B) pyramidal hippocampal neurons. (C) The average number of spikes elicited to one second long current injections of 0 pA to 40 pA in 5 pA increments from hippocampal pyramidal neurons expressing WT (black symbols, N=8) or T767I (red symbols, N=13) channels. The increase of the response for T767I expressing neurons was significant by ANOVA (p<0.05). Data presented as mean ± SEM.
Similar articles
- Characterization of a de novo SCN8A mutation in a patient with epileptic encephalopathy.
de Kovel CG, Meisler MH, Brilstra EH, van Berkestijn FM, van 't Slot R, van Lieshout S, Nijman IJ, O'Brien JE, Hammer MF, Estacion M, Waxman SG, Dib-Hajj SD, Koeleman BP. de Kovel CG, et al. Epilepsy Res. 2014 Nov;108(9):1511-8. doi: 10.1016/j.eplepsyres.2014.08.020. Epub 2014 Sep 4. Epilepsy Res. 2014. PMID: 25239001 Free PMC article. - Aberrant Sodium Channel Currents and Hyperexcitability of Medial Entorhinal Cortex Neurons in a Mouse Model of SCN8A Encephalopathy.
Ottolini M, Barker BS, Gaykema RP, Meisler MH, Patel MK. Ottolini M, et al. J Neurosci. 2017 Aug 9;37(32):7643-7655. doi: 10.1523/JNEUROSCI.2709-16.2017. Epub 2017 Jul 4. J Neurosci. 2017. PMID: 28676574 Free PMC article. - Neuronal hyperexcitability in a mouse model of SCN8A epileptic encephalopathy.
Lopez-Santiago LF, Yuan Y, Wagnon JL, Hull JM, Frasier CR, O'Malley HA, Meisler MH, Isom LL. Lopez-Santiago LF, et al. Proc Natl Acad Sci U S A. 2017 Feb 28;114(9):2383-2388. doi: 10.1073/pnas.1616821114. Epub 2017 Feb 13. Proc Natl Acad Sci U S A. 2017. PMID: 28193882 Free PMC article. - SCN8A Epilepsy, Developmental Encephalopathy, and Related Disorders.
Talwar D, Hammer MF. Talwar D, et al. Pediatr Neurol. 2021 Sep;122:76-83. doi: 10.1016/j.pediatrneurol.2021.06.011. Epub 2021 Aug 3. Pediatr Neurol. 2021. PMID: 34353676 Review. - SCN8A encephalopathy: Mechanisms and models.
Meisler MH. Meisler MH. Epilepsia. 2019 Dec;60 Suppl 3(Suppl 3):S86-S91. doi: 10.1111/epi.14703. Epilepsia. 2019. PMID: 31904118 Free PMC article. Review.
Cited by
- Parvalbumin interneuron impairment causes synaptic transmission deficits and seizures in SCN8A developmental and epileptic encephalopathy.
Miralles RM, Boscia AR, Kittur S, Hanflink JC, Panchal PS, Yorek MS, Deutsch TCJ, Reever CM, Vundela SR, Wengert ER, Patel MK. Miralles RM, et al. JCI Insight. 2024 Oct 22;9(20):e181005. doi: 10.1172/jci.insight.181005. JCI Insight. 2024. PMID: 39435659 Free PMC article. - The Node of Ranvier as an Interface for Axo-Glial Interactions: Perturbation of Axo-Glial Interactions in Various Neurological Disorders.
Dolma S, Joshi A. Dolma S, et al. J Neuroimmune Pharmacol. 2023 Jun;18(1-2):215-234. doi: 10.1007/s11481-023-10072-z. Epub 2023 Jun 7. J Neuroimmune Pharmacol. 2023. PMID: 37285016 Review. - SCN8A epileptic encephalopathy mutations display a gain-of-function phenotype and divergent sensitivity to antiepileptic drugs.
Guo QB, Zhan L, Xu HY, Gao ZB, Zheng YM. Guo QB, et al. Acta Pharmacol Sin. 2022 Dec;43(12):3139-3148. doi: 10.1038/s41401-022-00955-x. Epub 2022 Jul 27. Acta Pharmacol Sin. 2022. PMID: 35902765 Free PMC article. - A nutraceutical product, extracted from Cannabis sativa, modulates voltage-gated sodium channel function.
Milligan CJ, Anderson LL, Bowen MT, Banister SD, McGregor IS, Arnold JC, Petrou S. Milligan CJ, et al. J Cannabis Res. 2022 Jun 10;4(1):30. doi: 10.1186/s42238-022-00136-x. J Cannabis Res. 2022. PMID: 35689251 Free PMC article. - Genotype-phenotype correlations in SCN8A-related epilepsy: a cohort study of Chinese children in southern China.
Peng BW, Tian Y, Chen L, Duan LF, Wang XY, Zhu HX, Shi KL, Zheng KL, Shen HL, Liang W, Li XJ, Chen WX. Peng BW, et al. Brain. 2022 May 24;145(4):e24-e27. doi: 10.1093/brain/awac038. Brain. 2022. PMID: 35230384 Free PMC article. No abstract available.
References
- Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS, Matthews G. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron. 2001;30:91–104. - PubMed
- Burgess DL, Kohrman DC, Galt J, Plummer NW, Jones JM, Spear B, Meisler MH. Mutation of a new sodium channel gene, Scn8a, in the mouse mutant ‘motor endplate disease’. Nature Genetics. 1995;10:461–465. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous