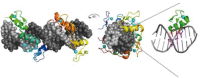Type II restriction endonucleases--a historical perspective and more - PubMed (original) (raw)
Review
. 2014 Jul;42(12):7489-527.
doi: 10.1093/nar/gku447. Epub 2014 May 30.
Affiliations
- PMID: 24878924
- PMCID: PMC4081073
- DOI: 10.1093/nar/gku447
Review
Type II restriction endonucleases--a historical perspective and more
Alfred Pingoud et al. Nucleic Acids Res. 2014 Jul.
Erratum in
- Type II restriction endonucleases - a historical perspective and more.
Pingoud A, Wilson GG, Wende W. Pingoud A, et al. Nucleic Acids Res. 2016 Sep 19;44(16):8011. doi: 10.1093/nar/gkw513. Epub 2016 Jun 7. Nucleic Acids Res. 2016. PMID: 27270082 Free PMC article. No abstract available.
Abstract
This article continues the series of Surveys and Summaries on restriction endonucleases (REases) begun this year in Nucleic Acids Research. Here we discuss 'Type II' REases, the kind used for DNA analysis and cloning. We focus on their biochemistry: what they are, what they do, and how they do it. Type II REases are produced by prokaryotes to combat bacteriophages. With extreme accuracy, each recognizes a particular sequence in double-stranded DNA and cleaves at a fixed position within or nearby. The discoveries of these enzymes in the 1970s, and of the uses to which they could be put, have since impacted every corner of the life sciences. They became the enabling tools of molecular biology, genetics and biotechnology, and made analysis at the most fundamental levels routine. Hundreds of different REases have been discovered and are available commercially. Their genes have been cloned, sequenced and overexpressed. Most have been characterized to some extent, but few have been studied in depth. Here, we describe the original discoveries in this field, and the properties of the first Type II REases investigated. We discuss the mechanisms of sequence recognition and catalysis, and the varied oligomeric modes in which Type II REases act. We describe the surprising heterogeneity revealed by comparisons of their sequences and structures.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
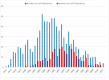
Figure 1.
Number of publications for EcoRI and EcoRV per year from 1972 to 2012. Only publications are listed in which EcoRI and EcoRV are listed in the title. Source: REBASE (7).
Figure 2.
Hamilton Smith and Daniel Nathans at the Nobel Prize press conference, 12 October 1978 (reproduced with permission from Susie Fitzhugh). Original Repository: Alan Mason Chesney Medical Archives, Daniel Nathans Collection.
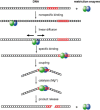
Figure 3.
Schematic illustration of the steps involved in DNA recognition and cleavage by REases (120).
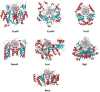
Figure 4.
Co-crystal structures of specific restriction enzyme–DNA complexes determined between 1990 and 1999.
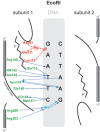
Figure 5.
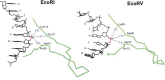
Schematic representation of the interaction of EcoRI with its recognition sequence. For clarity, interactions with only one subunit are shown; those with the other subunit are identical and symmetric. Hydrogen bonds and polar interactions are shown as arrows, van der Waals interactions as dotted lines. Amino acids and interactions involved in catalysis are depicted in red; those involved with sequence-recognition are depicted in green and blue (120).
Figure 6.
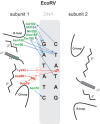
Schematic representation of the interaction of EcoRV with its recognition sequence. Interactions with only one subunit are shown; those with the other subunit are identical and symmetric. Amino acids and interactions involved in catalysis are depicted in red; those involved with sequence-recognition are depicted in green and blue (120).
Figure 7.
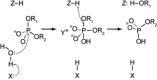
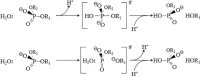
A general mechanism for DNA cleavage by EcoRI and EcoRV. An activated water molecule attacks the phosphorous in-line with the phosphodiester bond to be cleaved by an SN2 reaction, which proceeds with inversion of configuration. X, Y and Z are a general base, a Lewis acid and a general acid, respectively.
Figure 8.
The active site (PD…D/ExK) of EcoRI and EcoRV.
Figure 9.
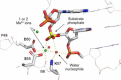
An example of a REase catalytic site (MvaI, pdb: 2OAA). The nucleophilic water is oriented with tetrahedral geometry to ‘attack’ the phosphorus: one H-bond is to K87 and one H-bond to the 3′-phosphate oxygen, both of which might act as the general base. One lone pair orbital of the attacking water is to the metal ion, and one lone pair orbital to the phosphorus atom.
Figure 10.
Alternative mechanisms of phosphoryl transfer reactions: associative (top) and dissociative (bottom). The mechanisms differ in the order of bond formation and breakage, and in the nature of the transition state (317).
Figure 11.
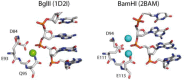
Comparison of the active sites of two structurally very similar restriction enzymes BglII (PDB 1D21, with one Na+ ion in the active center, which can be replaced by a Ca2+ ion by soaking) and BamHI (PDB 2BAM, with two Ca2+ ions in the active center) (313).
Figure 12.
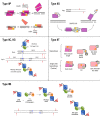
The subunit composition and cleavage mechanism of selected subtypes of Type II REases. Type IIP enzymes act mainly as homodimers (top), and cleave both DNA strands at once. Some act as dimers of dimers (homotetramers) instead, and do the same. Still others act as monomers (bottom) and cleave the DNA strands separately, one after the other. Bright triangles represent catalytic sites. Type IIS enzymes generally bind as monomers, but cleave as ‘transient’ homodimers. Type IIB enzymes cleave on both sides of their bipartite recognition sequences. Their subunit/domain stoichiometry and polypeptide chain continuity varies. Three examples of primary forms are shown: BcgI, AloI and HaeIV. These forms assemble in higher-order oligomers for cleavage. Type IIB enzymes display bilateral symmetry with respect to their methylation and cleavage positions. It is not clear whether they cleave to the left or to the right of the half-sequence bound. Type IIG enzymes (e.g. BcgI) might cleave upstream (left) of their bound recognition half-site. All other Type IIG enzymes (e.g. MmeI) cleave downstream from the site, often with the same geometry. These proteins have very similar amino acid sequences, however, suggesting that somehow the reactions are the same. Type IIT enzymes cleave within or close to asymmetric sequences. Composition varies; they have two different catalytic sites: top-strand specific and bottom-strand specific. In some, both subunits/domains interact with the recognition sequence (left cartoons). In others, only the larger subunit/domain recognizes the DNA.
Figure 13.
Mode of DNA binding by zinc finger proteins: each finger recognizes approximately three base pairs of the recognition sequence. For one zinc finger the amino acids forming essential base contacts (residues at positions 1, 2, 3, 6 of each helix) are shown in purple.
Figure 14.
Mode of DNA binding by a TAL effector: DNA binding is mediated by a central region comprising a series of near-identical tandem repeats, usually 34 residues in length. The amino acid residues at positions 12 and 13 are hyper-variable (the ‘repeat variable di-residue’, or RVD). The side chain of residue 13 of each repeat determines the base recognized by that repeat in a simple but ambiguous 1:1 ‘recognition code’. The repeats precisely track the sense strand of the DNA, and so the order of the repeats determines the bp sequence recognized. The RVD (HD) is shown in red.
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.
Elwenspoek MM, Thom H, Sheppard AL, Keeney E, O'Donnell R, Jackson J, Roadevin C, Dawson S, Lane D, Stubbs J, Everitt H, Watson JC, Hay AD, Gillett P, Robins G, Jones HE, Mallett S, Whiting PF. Elwenspoek MM, et al. Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article. - "I've Spent My Whole Life Striving to Be Normal": Internalized Stigma and Perceived Impact of Diagnosis in Autistic Adults.
Huang Y, Trollor JN, Foley KR, Arnold SRC. Huang Y, et al. Autism Adulthood. 2023 Dec 1;5(4):423-436. doi: 10.1089/aut.2022.0066. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116050 Free PMC article. - Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.
Love AMA, Edwards C, Cai RY, Gibbs V. Love AMA, et al. Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article. - Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.
Triana L, Palacios Huatuco RM, Campilgio G, Liscano E. Triana L, et al. Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
- Bacterial defense systems exhibit synergistic anti-phage activity.
Wu Y, Garushyants SK, van den Hurk A, Aparicio-Maldonado C, Kushwaha SK, King CM, Ou Y, Todeschini TC, Clokie MRJ, Millard AD, Gençay YE, Koonin EV, Nobrega FL. Wu Y, et al. Cell Host Microbe. 2024 Apr 10;32(4):557-572.e6. doi: 10.1016/j.chom.2024.01.015. Epub 2024 Feb 22. Cell Host Microbe. 2024. PMID: 38402614 - Genome-wide systematic identification of methyltransferase recognition and modification patterns.
Jensen TØ, Tellgren-Roth C, Redl S, Maury J, Jacobsen SAB, Pedersen LE, Nielsen AT. Jensen TØ, et al. Nat Commun. 2019 Aug 19;10(1):3311. doi: 10.1038/s41467-019-11179-9. Nat Commun. 2019. PMID: 31427571 Free PMC article. - Structure, subunit organization and behavior of the asymmetric Type IIT restriction endonuclease BbvCI.
Shen BW, Doyle L, Bradley P, Heiter DF, Lunnen KD, Wilson GG, Stoddard BL. Shen BW, et al. Nucleic Acids Res. 2019 Jan 10;47(1):450-467. doi: 10.1093/nar/gky1059. Nucleic Acids Res. 2019. PMID: 30395313 Free PMC article. - Isospecific adenine DNA methyltransferases show distinct preferences towards DNA substrates.
Wons E, Mruk I, Kaczorowski T. Wons E, et al. Sci Rep. 2018 May 29;8(1):8243. doi: 10.1038/s41598-018-26434-0. Sci Rep. 2018. PMID: 29844340 Free PMC article. - Kinetic Basis of the Bifunctionality of SsoII DNA Methyltransferase.
Timofeyeva NA, Ryazanova AY, Norkin MV, Oretskaya TS, Fedorova OS, Kubareva EA. Timofeyeva NA, et al. Molecules. 2018 May 16;23(5):1192. doi: 10.3390/molecules23051192. Molecules. 2018. PMID: 29772716 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases