UCHL1 deficiency exacerbates human islet amyloid polypeptide toxicity in β-cells: evidence of interplay between the ubiquitin/proteasome system and autophagy - PubMed (original) (raw)
UCHL1 deficiency exacerbates human islet amyloid polypeptide toxicity in β-cells: evidence of interplay between the ubiquitin/proteasome system and autophagy
Safia Costes et al. Autophagy. 2014 Jun.
Abstract
The islet in type 2 diabetes mellitus (T2DM) is characterized by a deficit in β-cells and increased β-cell apoptosis attributable at least in part to intracellular toxic oligomers of IAPP (islet amyloid polypeptide). β-cells of individuals with T2DM are also characterized by accumulation of polyubiquitinated proteins and deficiency in the deubiquitinating enzyme UCHL1 (ubiquitin carboxyl-terminal esterase L1 [ubiquitin thiolesterase]), accounting for a dysfunctional ubiquitin/proteasome system. In the present study, we used mouse genetics to elucidate in vivo whether a partial deficit in UCHL1 enhances the vulnerability of β-cells to human-IAPP (hIAPP) toxicity, and thus accelerates diabetes onset. We further investigated whether a genetically induced deficit in UCHL1 function in β-cells exacerbates hIAPP-induced alteration of the autophagy pathway in vivo. We report that a deficit in UCHL1 accelerated the onset of diabetes in hIAPP transgenic mice, due to a decrease in β-cell mass caused by increased β-cell apoptosis. We report that UCHL1 dysfunction aggravated the hIAPP-induced defect in the autophagy/lysosomal pathway, illustrated by the marked accumulation of autophagosomes and cytoplasmic inclusions positive for SQSTM1/p62 and polyubiquitinated proteins with lysine 63-specific ubiquitin chains. Collectively, this study shows that defective UCHL1 function may be an early contributor to vulnerability of pancreatic β-cells for protein misfolding and proteotoxicity, hallmark defects in islets of T2DM. Also, given that deficiency in UCHL1 exacerbated the defective autophagy/lysosomal degradation characteristic of hIAPP proteotoxicity, we demonstrate a previously unrecognized role of UCHL1 in the function of the autophagy/lysosomal pathway in β-cells.
Keywords: SQSTM1/p62; apoptosis; autophagy; diabetes; islet amyloid polypeptide; ubiquitin carboxyl-terminal esterase L1; β-cell.
Figures
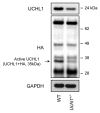
Figure 1. UCHL1 activity and expression are decreased in islets isolated from Uchl1nm3419 heterozygous mice (Uchl1+/−). Activity of UCHL1 was assessed by active-site labeling of deubiquitinating enzymes. Islet obtained from 8–10-wk-old WT and Uchl1+/− mice were incubated with HA-Ub-VS and lysates were analyzed by western blotting using anti-HA antibody. Levels of UCHL1 were analyzed by western blotting. Levels of GAPDH were shown as loading control.
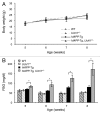
Figure 2. UCHL1 deficiency accelerates diabetes progression in _hIAPP_-Tg mice. Body weight (A) and fasting blood glucose (B) were measured in the 4 groups of mice: wild-type (WT: n = 7); UCHL1 deficient (Uchl1+/−: n = 9), hIAPP transgenic (_hIAPP_-Tg: n = 9) and hIAPP transgenic mice deficient for UCHL1 (_hIAPP_-Tg, Uchl1+/−: n = 17 at 5, 6, and 7 wk; n = 7 at 8 wk). Data are expressed as mean ± SEM; *P < 0.05.
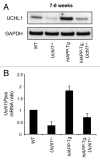
Figure 3.Uchl1 mRNA and UCHL1 protein levels in mouse islets. (A) UCHL1 protein levels were assessed by western blotting using islet protein lysates obtained from 7–8-wk-old WT, Uchl1+/−, _hIAPP_-Tg, and _hIAPP_-Tg, Uchl1+/− mice (n = 3). GAPDH was used as a control. (B) Levels of Uchl1 mRNA were evaluated by RT-qPCR in islets isolated from 7–8-wk-old WT, Uchl1+/−, _hIAPP_-Tg, and _hIAPP_-Tg, Uchl1+/− mice (n = 2–4). Data are expressed as mean ± SEM. The mouse Ppia (peptidylprolyl isomerase A [cyclophilin A]) gene was used for the ratio.
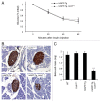
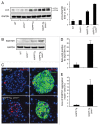
Figure 4. Deficiency in UCHL1 decreases β-cell mass but does not alter insulin sensitivity in _hIAPP_-Tg mice. (A) Insulin tolerance tests were performed on 7-wk-old mice of the indicated genotypes: _hIAPP_-Tg (n = 10) and _hIAPP_-Tg, Uchl1+/− mice (n = 14). Results represent the blood glucose concentration as a percentage of the starting glucose value and are expressed as mean ± SEM (B) Representative pancreatic islets immunostained for insulin (brown) and counterstained with hematoxylin (blue) from WT; Uchl1+/−; _hIAPP_-Tg; and _hIAPP_-Tg, Uchl1+/− mice. (C) β-cell mass was evaluated in the 4 groups of 7–8-wk-old mice: WT (n = 4), Uchl1+/− (n = 4), _hIAPP_-Tg (n = 3) and _hIAPP_-Tg, Uchl1+/− mice (n = 4). Data are expressed as mean ± SEM; ***P < 0.001, significant differences vs. _hIAPP_-Tg mice.
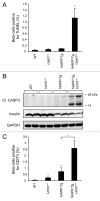
Figure 5. UCHL1 deficiency exacerbates β-cell ER stress and apoptosis in _hIAPP_-Tg mice. (A) β-cell apoptosis (percentage of β-cells positive for TUNEL) was evaluated in 7–8-wk-old WT (n = 3), Uchl1+/− (n = 3), _hIAPP_-Tg (n = 3) and _hIAPP_-Tg, Uchl1+/− mice (n = 3). (B) Protein levels of cleaved CASP3 (Cl. CASP3) were assessed by western blotting using islet protein lysates obtained from 7–8-wk-old WT (n = 2), Uchl1+/− (n = 4), _hIAPP_-Tg (n = 4) and _hIAPP_-Tg, Uchl1+/− mice (n = 5). Insulin and GAPDH were used as controls. (C) β-cell ER stress (percentage of β-cells positive for nuclear DDIT3) was evaluated in 7–8-wk-old WT (n = 3), Uchl1+/− (n = 3), _hIAPP_-Tg (n = 3) and _hIAPP_-Tg, Uchl1+/− mice (n = 3). Data are expressed as mean ± SEM; *P < 0.05, significant differences vs. _hIAPP_-Tg mice.
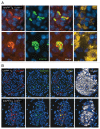
Figure 6. UCHL1 deficiency exacerbates defects in lysosomal degradation in _hIAPP_-Tg mouse islets. Protein levels of LC3 (A) and SQSTM1 (B) were assessed by western blotting using islet protein lysates obtained from 7–8-wk-old WT (n = 2), Uchl1+/− (n = 2), _hIAPP_-Tg (n = 2) and _hIAPP_-Tg, Uchl1+/− mice (n = 3). GAPDH was used as a control. The graph represents the quantification of LC3-II protein levels. (C) SQSTM1 protein levels were assessed by immunofluorescence (SQSTM1, red; insulin, green; nuclei, blue) in pancreatic tissue from 7–8-wk-old _hIAPP_-Tg mice and _hIAPP_-Tg, Uchl1+/− mice. (D) The graph represents the quantification of β-cells positive for SQSTM1 in each group (expressed in %). (E) The graph represents the quantification of β-cell area positive for SQSTM1 aggregates in each group (expressed in %). Data are expressed as mean ± SEM; **P < 0.01; ***P < 0.001, significant differences vs. _hIAPP_-Tg mice.
Figure 7. (A) Fluorescence confocal images of LC3 and SQSTM1 at magnification x 63 (LC3, red; SQSTM1, green; insulin, yellow; nuclei, blue) in pancreatic tissue from 7–8-wk-old _hIAPP_-Tg, Uchl1+/− mice. (B) The detection and localization of ubiquitin lysine 63 chains were assessed by immunofluorescence (ubiquitin K63, red; SQSTM1, green; insulin, white; nuclei, blue) in pancreatic tissue from 7–8-wk-old _hIAPP_-Tg mice and _hIAPP_-Tg, Uchl1+/− mice.
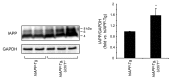
Figure 8. UCHL1 deficiency increases IAPP/proIAPP protein levels in _hIAPP_-Tg mouse islets. Protein levels of IAPP were assessed by western blotting using islet protein lysates obtained from 7–8-wk-old _hIAPP_-Tg mice (n = 3) and _hIAPP_-Tg, Uchl1+/− mice (n = 5). GAPDH was used as a control. The slowly migrating bands represent unprocessed and partially processed proIAPP (8 and 6 kDa, respectively). The graph represents the quantification of IAPP protein levels. Data are expressed as mean ± SEM; *P < 0.05.
Similar articles
- β-cell dysfunctional ERAD/ubiquitin/proteasome system in type 2 diabetes mediated by islet amyloid polypeptide-induced UCH-L1 deficiency.
Costes S, Huang CJ, Gurlo T, Daval M, Matveyenko AV, Rizza RA, Butler AE, Butler PC. Costes S, et al. Diabetes. 2011 Jan;60(1):227-38. doi: 10.2337/db10-0522. Epub 2010 Oct 27. Diabetes. 2011. PMID: 20980462 Free PMC article. - Amyloidogenic peptide oligomer accumulation in autophagy-deficient β cells induces diabetes.
Kim J, Cheon H, Jeong YT, Quan W, Kim KH, Cho JM, Lim YM, Oh SH, Jin SM, Kim JH, Lee MK, Kim S, Komatsu M, Kang SW, Lee MS. Kim J, et al. J Clin Invest. 2014 Aug;124(8):3311-24. doi: 10.1172/JCI69625. Epub 2014 Jul 18. J Clin Invest. 2014. PMID: 25036705 Free PMC article. - Human IAPP-induced pancreatic β cell toxicity and its regulation by autophagy.
Shigihara N, Fukunaka A, Hara A, Komiya K, Honda A, Uchida T, Abe H, Toyofuku Y, Tamaki M, Ogihara T, Miyatsuka T, Hiddinga HJ, Sakagashira S, Koike M, Uchiyama Y, Yoshimori T, Eberhardt NL, Fujitani Y, Watada H. Shigihara N, et al. J Clin Invest. 2014 Aug;124(8):3634-44. doi: 10.1172/JCI69866. Epub 2014 Jul 18. J Clin Invest. 2014. PMID: 25036706 Free PMC article. - Human IAPP amyloidogenic properties and pancreatic β-cell death.
Fernández MS. Fernández MS. Cell Calcium. 2014 Nov;56(5):416-27. doi: 10.1016/j.ceca.2014.08.011. Epub 2014 Aug 27. Cell Calcium. 2014. PMID: 25224501 Review. - The β-cell assassin: IAPP cytotoxicity.
Raleigh D, Zhang X, Hastoy B, Clark A. Raleigh D, et al. J Mol Endocrinol. 2017 Oct;59(3):R121-R140. doi: 10.1530/JME-17-0105. Epub 2017 Aug 15. J Mol Endocrinol. 2017. PMID: 28811318 Review.
Cited by
- Connecting the Dots in the Neuroglobin-Protein Interaction Network of an Unstressed and Ferroptotic Cell Death Neuroblastoma Model.
Van Acker ZP, Van Raemdonck GA, Logie E, Van Acker SI, Baggerman G, Vanden Berghe W, Ponsaerts P, Dewilde S. Van Acker ZP, et al. Cells. 2019 Aug 11;8(8):873. doi: 10.3390/cells8080873. Cells. 2019. PMID: 31405213 Free PMC article. - Molecular Mechanisms of Amylin Turnover, Misfolding and Toxicity in the Pancreas.
Bhowmick DC, Kudaibergenova Z, Burnett L, Jeremic AM. Bhowmick DC, et al. Molecules. 2022 Feb 2;27(3):1021. doi: 10.3390/molecules27031021. Molecules. 2022. PMID: 35164285 Free PMC article. Review. - Downregulated UCHL1 Accelerates Gentamicin-Induced Auditory Cell Death via Autophagy.
Kim YJ, Kim K, Lee YY, Choo OS, Jang JH, Choung YH. Kim YJ, et al. Mol Neurobiol. 2019 Nov;56(11):7433-7447. doi: 10.1007/s12035-019-1598-y. Epub 2019 Apr 30. Mol Neurobiol. 2019. PMID: 31041655 - Autophagy and Alzheimer's Disease: From Molecular Mechanisms to Therapeutic Implications.
Uddin MS, Stachowiak A, Mamun AA, Tzvetkov NT, Takeda S, Atanasov AG, Bergantin LB, Abdel-Daim MM, Stankiewicz AM. Uddin MS, et al. Front Aging Neurosci. 2018 Jan 30;10:04. doi: 10.3389/fnagi.2018.00004. eCollection 2018. Front Aging Neurosci. 2018. PMID: 29441009 Free PMC article. Review. - Human islet amyloid polypeptide: A therapeutic target for the management of type 2 diabetes mellitus.
Roham PH, Save SN, Sharma S. Roham PH, et al. J Pharm Anal. 2022 Aug;12(4):556-569. doi: 10.1016/j.jpha.2022.04.001. Epub 2022 Apr 7. J Pharm Anal. 2022. PMID: 36105173 Free PMC article. Review.
References
- Gurlo T, Ryazantsev S, Huang CJ, Yeh MW, Reber HA, Hines OJ, O’Brien TD, Glabe CG, Butler PC. Evidence for proteotoxicity in beta cells in type 2 diabetes: toxic islet amyloid polypeptide oligomers form intracellularly in the secretory pathway. Am J Pathol. 2010;176:861–9. doi: 10.2353/ajpath.2010.090532. - DOI - PMC - PubMed
- Huang CJ, Haataja L, Gurlo T, Butler AE, Wu X, Soeller WC, Butler PC. Induction of endoplasmic reticulum stress-induced beta-cell apoptosis and accumulation of polyubiquitinated proteins by human islet amyloid polypeptide. Am J Physiol Endocrinol Metab. 2007;293:E1656–62. doi: 10.1152/ajpendo.00318.2007. - DOI - PubMed
- Huang CJ, Lin CY, Haataja L, Gurlo T, Butler AE, Rizza RA, Butler PC. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes. 2007;56:2016–27. doi: 10.2337/db07-0197. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous