Mutations of CEP83 cause infantile nephronophthisis and intellectual disability - PubMed (original) (raw)
. 2014 Jun 5;94(6):905-14.
doi: 10.1016/j.ajhg.2014.05.002. Epub 2014 May 29.
Heon Yung Gee 2, Pauline Krug 3, Kwangsic Joo 4, Jan Halbritter 2, Lilya Belkacem 1, Emilie Filhol 1, Jonathan D Porath 2, Daniela A Braun 2, Markus Schueler 2, Amandine Frigo 1, Olivier Alibeu 5, Cécile Masson 6, Karine Brochard 7, Bruno Hurault de Ligny 8, Robert Novo 9, Christine Pietrement 10, Hulya Kayserili 11, Rémi Salomon 12, Marie-Claire Gubler 3, Edgar A Otto 13, Corinne Antignac 14, Joon Kim 4, Alexandre Benmerah 1, Friedhelm Hildebrandt 15, Sophie Saunier 16
Affiliations
- PMID: 24882706
- PMCID: PMC4121475
- DOI: 10.1016/j.ajhg.2014.05.002
Mutations of CEP83 cause infantile nephronophthisis and intellectual disability
Marion Failler et al. Am J Hum Genet. 2014.
Abstract
Ciliopathies are a group of hereditary disorders associated with defects in cilia structure and function. The distal appendages (DAPs) of centrioles are involved in the docking and anchoring of the mother centriole to the cellular membrane during ciliogenesis. The molecular composition of DAPs was recently elucidated and mutations in two genes encoding DAPs components (CEP164/NPHP15, SCLT1) have been associated with human ciliopathies, namely nephronophthisis and orofaciodigital syndrome. To identify additional DAP components defective in ciliopathies, we independently performed targeted exon sequencing of 1,221 genes associated with cilia and 5 known DAP protein-encoding genes in 1,255 individuals with a nephronophthisis-related ciliopathy. We thereby detected biallelic mutations in a key component of DAP-encoding gene, CEP83, in seven families. All affected individuals had early-onset nephronophthisis and four out of eight displayed learning disability and/or hydrocephalus. Fibroblasts and tubular renal cells from affected individuals showed an altered DAP composition and ciliary defects. In summary, we have identified mutations in CEP83, another DAP-component-encoding gene, as a cause of infantile nephronophthisis associated with central nervous system abnormalities in half of the individuals.
Copyright © 2014 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
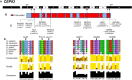
Identification of Ten Different CEP83 Mutations in Seven Families with NPHP-RC (A) Exon structure of human CEP83 cDNA (RefSeq NM_016122.2). Positions of start codon (ATG) and of stop codon (TGA) are indicated in exons 3 and 17, respectively. (B) Domain structure of the respective protein. CEP83 contains multiple coiled-coil (red) domains. (C) Ten homozygous or compound-heterozygous CEP83 mutations. For the mutations detected, black arrows indicate positions in relation to exons and protein domains. Except for the c.335_352del mutation, all mutations concern amino acids found in coiled-coil domains, mostly in the N-terminal and C-terminal parts of the protein. Family numbers are underlined and predicted translational changes are indicated. Affected individuals had at least one missense mutation and a deletion. (D) Partial protein alignment of CEP83 shows evolutionary conservation of the amino acids affected by the identified mutations (p.Leu87Pro, p.Pro112_Leu117del, p.Arg511Pro, p.Glu684del, and p.Gln692del).
Figure 2
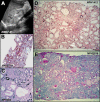
Sonographic and Histological Lesions in Individuals with CEP83 Mutations (A) Renal ultrasound of individual A4037-21 shows corticomedullary cysts and increased echogenicity. (B–E) Renal biopsy findings in individuals A4037-21 (B), NPH982 (C), NPH1412 (D), and NPH2036 (E). (B) Tubular dilatations are associated with diffuse interstitial fibrosis in individual A4037-21. Trichrome light green. (C–E) Numerous glomeruli are preserved and others show a retracted glomerular tuft surrounded by a thickened capsular basement membrane. (C) Microcystic tubular dilatations are associated with foci of tubular atrophy and intersitial fibrosis in individual NPH982. (D) In individual NPH1412, most cortical tubules are dilated and surrounded by interstitial fibrosis. (C and D) Silver impregnation. (E) Diffuse tubular atrophy, massive interstitial fibrosis, and focal interstitial infiltration are associated in individual NPH2036. PAS.
Figure 3
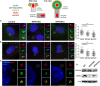
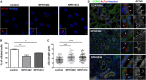
Individuals Carrying CEP83 Mutations Show Abnormal DAP Organization Top: Schematic representation of the structural organization of the centrosome where the localization of different markers of centriolar subdomains (distal [DAPs] and subdistal [SDAPs]) are stressed. (A–C) Fibroblasts from control or affected individuals (NPH1402 and NPH1412) were processed for immunofluorescence with antibodies against centrin (A and B, red; mouse monoclonal [Millipore, 04-1624]) to stain centrioles and CEP83 (A, green; rabbit polyclonal [Sigma, HPA038161]) or CEP164 (B, green; rabbit polyclonal [Sigma, HPA037606]) or with antibodies against ODF2 (C, red; mouse monoclonal antibody [clone 1A1, Novus Biological]) to stain SDAPs and CEP164 (C, green). Insets on the right show higher magnification of representative centrosomes indicated by a white square in the corresponding images. Arrows indicate CEP83 or CEP164 staining. The intensity of CEP83 (A) and CEP164 (B) staining at the centrosome was quantified by ImageJ (normalized arbitrary units, one representative experiment out of three [∗∗∗p < 0.0001], were calculated via Dunn’s Multiple Comparison Test after the analysis of variance ANOVA test) and shows a reduction in intensity of either CEP83 or CEP164 in affected individual cells compared to control. (C) Samples were imaged with an n-SIM microscope (Nikon). Acquisitions were performed in 3D SIM mode, before image reconstruction with the NIS-Elements software (Nikon) based on Gustafsson et al. Scale bars represent 5 μm. (D) Cell lysates from fibroblasts were collected and protein levels of CEP83, CEP164, and γ-tubulin (mouse monoclonal [Sigma, T5326]) were analyzed by immunoblotting with indicated antibodies. CEP83 level is decreased in affected individuals fibroblasts compared to control and CEP164 level remains the same.
Figure 4
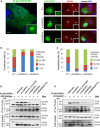
CEP83 Mutations Affect Centrosomal Localization and Interaction of CEP83 with CEP164 (A) RPE1 cells were transiently transfected (Lipofectamine LTX, Life Technologies) with plasmids encoding for FLAG-tagged wild-type (WT) or c.2007del (p.Glu669Aspfs∗14) or c.2075_2077del (p.Gln692del) CEP83 variants, fixed, and stained for FLAG (green; mouse monoclonal [Sigma, F3165]) and CEP164 (red). Scale bar represents 5 μm. (B and C) Levels of CEP164 (C) and FLAG (CEP83, B) staining at the centrosome were quantified with ImageJ. Centriolar CEP164 intensity is decreased in cells transfected with CEP83 WT compared to cells transfected with CEP83 p.Glu669Aspfs∗14 and p.Gln692del variants (arrows). (D and E) NIH 3T3 cells were transiently cotransfected with plasmids encoding for FLAG-tagged WT and variant forms of CEP83, Cep164-myc, and IFT20-GFP. Cell lysates were immunoprecipitated with an anti-FLAG antibody (mouse monoclonal, Sigma, M2). Cell lysates (bottom) and immunoprecipitates (IP, top) were analyzed by immunoblotting with antibodies against myc (CEP164, rabbit polyclonal [Santa Cruz Biotechnology, C-19]), FLAG (CEP83, rabbit polyclonal [Cell Signaling Technology, #2368]), and GFP (IFT20, rabbit polyclonal [Life Technologies, A-6455]) as indicated. Note that three C-terminal CEP83 variants affected the interaction with CEP164 and IFT120, whereas two N-terminal CEP83 variants did not.
Figure 5
Fibroblasts from Individuals with CEP83 Mutations Show Mild Ciliary Defects (A) Fibroblasts from control and affected individuals (NPH1402 and NPH14112) were grown on coverslips, serum starved for 48 hr, then fixed and processed for immunofluorescence with antibodies against acetylated tubulin (AcTub, red) to stain cilia. Insets show higher magnification of representative cilia. Scale bar represents 5 μm. (B) The ability of cells to form cilia, cells with a unique rod like AcTub staining, was quantified from images obtained in (A). Graphs show the mean ± SEM of at least three independent experiments in which at least 100 cells were counted. (C) The length of cilia was measured (μm) based on AcTub staining and the results for one representative experiment out of three are shown. (B and C) ∗p < 0.01, ∗∗p < 0.001, and ∗∗∗p < 0.0001 were calculated via Dunn’s Multiple Comparison Test after the analysis of variance ANOVA test. Ciliogenesis is reducted in fibroblasts of affected individuals which present longer cilia. (D) Sections of kidney biopsies from control, NPH1402, and NPH2036 individuals were stained for CEP83 (green) and AcTub (red). Nuclei were stained with Hoechst (blue). Higher magnifications of representative tubules regions are shown on the right panels and cilia are indicated by white arrows. Cilia present in the lumen of tubules are longer in affected individuals biopsies compared to control.
Similar articles
- The Role of Centrosome Distal Appendage Proteins (DAPs) in Nephronophthisis and Ciliogenesis.
Mansour F, Boivin FJ, Shaheed IB, Schueler M, Schmidt-Ott KM. Mansour F, et al. Int J Mol Sci. 2021 Nov 12;22(22):12253. doi: 10.3390/ijms222212253. Int J Mol Sci. 2021. PMID: 34830133 Free PMC article. Review. - Centriole distal appendages promote membrane docking, leading to cilia initiation.
Tanos BE, Yang HJ, Soni R, Wang WJ, Macaluso FP, Asara JM, Tsou MF. Tanos BE, et al. Genes Dev. 2013 Jan 15;27(2):163-8. doi: 10.1101/gad.207043.112. Genes Dev. 2013. PMID: 23348840 Free PMC article. - Super-resolution architecture of mammalian centriole distal appendages reveals distinct blade and matrix functional components.
Yang TT, Chong WM, Wang WJ, Mazo G, Tanos B, Chen Z, Tran TMN, Chen YD, Weng RR, Huang CE, Jane WN, Tsou MB, Liao JC. Yang TT, et al. Nat Commun. 2018 May 22;9(1):2023. doi: 10.1038/s41467-018-04469-1. Nat Commun. 2018. PMID: 29789620 Free PMC article. - Beyond nephronophthisis: Retinal dystrophy in the absence of kidney dysfunction in childhood expands the clinical spectrum of CEP83 deficiency.
Veldman BCF, Kuper WFE, Lilien M, Schuurs-Hoeijmakers JHM, Marcelis C, Phan M, Hettinga Y, Talsma HE, van Hasselt PM, Haijes HA. Veldman BCF, et al. Am J Med Genet A. 2021 Jul;185(7):2204-2210. doi: 10.1002/ajmg.a.62225. Epub 2021 May 3. Am J Med Genet A. 2021. PMID: 33938610 Free PMC article. - Ciliopathies and the Kidney: A Review.
McConnachie DJ, Stow JL, Mallett AJ. McConnachie DJ, et al. Am J Kidney Dis. 2021 Mar;77(3):410-419. doi: 10.1053/j.ajkd.2020.08.012. Epub 2020 Oct 9. Am J Kidney Dis. 2021. PMID: 33039432 Review.
Cited by
- Results of targeted next-generation sequencing in children with cystic kidney diseases often change the clinical diagnosis.
Obeidova L, Seeman T, Fencl F, Blahova K, Hojny J, Elisakova V, Reiterova J, Stekrova J. Obeidova L, et al. PLoS One. 2020 Jun 23;15(6):e0235071. doi: 10.1371/journal.pone.0235071. eCollection 2020. PLoS One. 2020. PMID: 32574212 Free PMC article. - Primary cilia and actin regulatory pathways in renal ciliopathies.
Kalot R, Sentell Z, Kitzler TM, Torban E. Kalot R, et al. Front Nephrol. 2024 Jan 16;3:1331847. doi: 10.3389/fneph.2023.1331847. eCollection 2023. Front Nephrol. 2024. PMID: 38292052 Free PMC article. Review. - The Role of Centrosome Distal Appendage Proteins (DAPs) in Nephronophthisis and Ciliogenesis.
Mansour F, Boivin FJ, Shaheed IB, Schueler M, Schmidt-Ott KM. Mansour F, et al. Int J Mol Sci. 2021 Nov 12;22(22):12253. doi: 10.3390/ijms222212253. Int J Mol Sci. 2021. PMID: 34830133 Free PMC article. Review. - Mutations in TRAF3IP1/IFT54 reveal a new role for IFT proteins in microtubule stabilization.
Bizet AA, Becker-Heck A, Ryan R, Weber K, Filhol E, Krug P, Halbritter J, Delous M, Lasbennes MC, Linghu B, Oakeley EJ, Zarhrate M, Nitschké P, Garfa-Traore M, Serluca F, Yang F, Bouwmeester T, Pinson L, Cassuto E, Dubot P, Elshakhs NAS, Sahel JA, Salomon R, Drummond IA, Gubler MC, Antignac C, Chibout S, Szustakowski JD, Hildebrandt F, Lorentzen E, Sailer AW, Benmerah A, Saint-Mezard P, Saunier S. Bizet AA, et al. Nat Commun. 2015 Oct 21;6:8666. doi: 10.1038/ncomms9666. Nat Commun. 2015. PMID: 26487268 Free PMC article. - Identification of 30 transition fibre proteins in Trypanosoma brucei reveals a complex and dynamic structure.
Ahmed M, Wheeler R, Týč J, Shafiq S, Sunter J, Vaughan S. Ahmed M, et al. J Cell Sci. 2024 May 15;137(10):jcs261692. doi: 10.1242/jcs.261692. Epub 2024 Jun 3. J Cell Sci. 2024. PMID: 38572631 Free PMC article.
References
- Satir P., Christensen S.T. Overview of structure and function of mammalian cilia. Annu. Rev. Physiol. 2007;69:377–400. - PubMed
- Delous M., Gaudé H.M., Saunier S. Genetic bases and pathogenic mechanisms of nephronophthisis. Drug Discov. Today Dis. Mech. 2013 Published online November 5, 2013.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous