Cisplatin-resistant cells in malignant pleural mesothelioma cell lines show ALDH(high)CD44(+) phenotype and sphere-forming capacity - PubMed (original) (raw)
Cisplatin-resistant cells in malignant pleural mesothelioma cell lines show ALDH(high)CD44(+) phenotype and sphere-forming capacity
Lourdes Cortes-Dericks et al. BMC Cancer. 2014.
Abstract
Background: Conventional chemotherapy in malignant pleural mesothelioma (MPM) has minimal impact on patient survival due to the supposed chemoresistance of cancer stem cells (CSCs). We sought to identify a sub-population of chemoresistant cells by using putative CSC markers, aldehyde dehydrogenase (ALDH) and CD44 in three MPM cell lines; H28, H2052 and Meso4.
Methods: The Aldefluor assay was used to measure ALDH activity and sort ALDH(high) and ALDH(low) cells. Drug-resistance was evaluated by cell viability, anchorage-independent sphere formation, flow-cytometry and qRT-PCR analyses.
Results: The ALDH(high) - and ALDH(low) -sorted fractions were able to demonstrate phenotypic heterogeneity and generate spheres, the latter being less efficient, and both showed an association with CD44. Cis- diamminedichloroplatinum (II) (cisplatin) treatment failed to reduce ALDH activity and conferred only a short-term inhibition of sphere generation in both ALDH(high) and ALDH(low) fractions of the three MPM cell lines. Induction of drug sensitivity by an ALDH inhibitor, diethylaminobenzaldehyde (DEAB) resulted in significant reductions in cell viability but not a complete elimination of the sphere-forming cells, suggestive of the presence of a drug-resistant subpopulation. At the transcript level, the cisplatin + DEAB-resistant cells showed upregulated mRNA expression levels for ALDH1A2, ALDH1A3 isozymes and CD44 indicating the involvement of these markers in conferring chemoresistance in both ALDH(high) and ALDH(low) fractions of the three MPM cell lines.
Conclusions: Our study shows that ALDH(high) CD44(+) cells are implicated in conveying tolerance to cisplatin in the three MPM cell lines. The combined use of CD44 and ALDH widens the window for identification and targeting of a drug-resistant population which may improve the current treatment modalities in mesothelioma.
Figures
Figure 1
MPM cell lines contain sphere-forming cell population. (A) H28, H2052 and Meso4 grown under anchorage-independent culture conditions at 5000 cells/ml/well generated spheres of varying sizes and showed different sphere-forming efficiencies. (B) Representative images of sphere formation taken on day 7 on three consecutive generations.
Figure 2
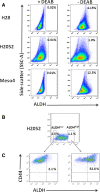
Detection of ALDH activity and ALDH high CD44 + cells within the ALDH high and ALDH low fractions of the three MPM cell lines. (A) Representative FACS analysis of ALDH activity in H28, H2052 and Meso4 using Aldefluor assay. Baseline control of ALDH fluorescence was established by the addition of ALDH inhibitor, DEAB (+DEAB) and used to provide the ALDHhigh region for cells without DEAB (- DEAB). The same gating principle was applied to sort for ALDHhigh cells. (B) Representative FACS-based sorting of ALDHhigh and ALDHlow fractions for H2052. The non-viable cells (PI+ cells) were excluded before gating the ALDHhigh cells. In all cases, ALDHlow cells represent <4% of the dimmest cells relative to the analysed population. Average purity of ALDHhigh cells was ≥98%. (C) The freshly-sorted ALDHhigh and ALDHlow fractions were immediately re-stained with CD44 APC-H7 antibody to determine the co-expression of ALDH and CD44. The same procedures were employed for ALDH sorting of H28 and H2052 cell lines and to determine the co-expression of ALDH and CD44. Three independent experiments were done for each cell line.
Figure 3
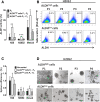
Effect of ALDH sorting on ALDH activity and sphere-forming efficiency in H28, H2052 and Meso4 cell lines after in vitro expansion. (A) The ALDH activity of the in vitro-expanded ALDHhigh and ALDHlow fractions was determined by Aldefluor assay. P0 represents the ALDH activity of the non-sorted cell lines, whereas P1-P4 accounts for the average ALDH activity measured after 4 passages of in vitro expansion. (B) Representative FACS analyses of ALDH activity of ALDH-sorted fractions of H2052 measured at every passage. (C) The sphere-forming efficiency was also assessed after the in vitro expansion of the ALDHhigh and ALDHlow fractions. Cells were grown under anchorage-independent cell culture conditions and were counted on day 7 for all cell lines and were used to determine the sphere-forming efficiency as described in the Methods. (D) Representative images of the sphere formation of ALDH-sorted fractions of H2052 taken at every passage. Results reflect the means and SDs of 3 independent experiments for each cell line. Results were statistically significant if p <0.05 (*p <0.05, **p <0.01).
Figure 4
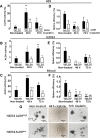
Effect of cisplatin treatment on ALDH activity and sphere-forming efficiency. Cells in 10 cm dishes were treated with the previously determined IC50 of cisplatin for ALDH-sorted fractions of the MPM cell lines. After the 48- and 72-h cisplatin treatments of ALDHhigh- and ALDHlow-sorted cells, ALDH activity (A-C) was determined on surviving cells and compared with the non-treated cells to evaluate the effect of cisplatin by flow cytometry. The sphere-forming efficiency of the cisplatin-resistant cells was also evaluated as described in the Methods, and compared with the non-treated cells (D-F). Results represent the means and SDs of 3 independent experiments each. Data are statistically significant if p <0.05 (*p <0.05, **p <0.01, ***p <0.001). Representative images of spheres from non-treated and cisplatin-treated H2052 ALDHhigh - and H2052 ALDHlow -sorted cells (G).
Figure 5
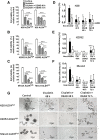
Cisplatin + DEAB treatment decreases cell viability and induces a short-term inhibition of sphere formation. ALDHhigh- and ALDHlow-sorted cells of H28, H2052 and Meso4 were pre-treated with 100 μM DEAB for 48 h, then re-incubated with cisplatin (Cis) for either 48 h or 72 h. Cell viability of the surviving cells was quantified using tryphan blue exclusion test which was performed in triplicates in 3 independent experiments (A-C). Surviving cells were allowed to form spheres under an anchorage-independent culture condition which were evaluated on day 7 and then compared with the non-treated cells (D-F). Results represent means and SDs of 3 experiments with 6 replicates each. Dashed lines symbolize 0 values. The level of significance was set at p <0.05 (*p <0.05, **p <0.01, ***p <0.001). Representative images of sphere-formation of ALDHhigh -sorted MPM cell lines, with and without cisplatin treatment, and in the presence or absence of 100 μM DEAB (G). The ALDHlow -sorted fraction of each cell line showed the same effect (images not shown).
Figure 6
Cisplatin + DEAB-resistant cells show increased mRNA levels of ALDH isozymes and CD44. mRNA was isolated from non-treated and surviving cells of the ALDH-sorted fractions of H28, H2052 and Meso4 after cisplatin treatment in the presence or absence of DEAB for 48 and 72 h. Relative mRNA expression levels of ALDH1A2 and ALDH1A3 (A-C) and CD44 (D-F) were determined by qRT-PCR using the ΔΔCT method. Histograms represent the means and SDs from 3 independent experiments each. Results are statistically significant if p <0.05 (*p <0.05, ***p <0.001).
Similar articles
- [Increased expression of acetaldehyde dehydrogenase in cisplatin-resistant human lung adenocarcinoma A549/DDP cells].
He J, Song X, Yu L, Li J, Qiao Z, Jiu R, Yu B, Liu X. He J, et al. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015 May;31(5):625-9. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015. PMID: 25940289 Chinese. - Putative cancer stem cells in malignant pleural mesothelioma show resistance to cisplatin and pemetrexed.
Cortes-Dericks L, Carboni GL, Schmid RA, Karoubi G. Cortes-Dericks L, et al. Int J Oncol. 2010 Aug;37(2):437-44. doi: 10.3892/ijo_00000692. Int J Oncol. 2010. PMID: 20596671 - A STAT3-NFkB/DDIT3/CEBPβ axis modulates ALDH1A3 expression in chemoresistant cell subpopulations.
Canino C, Luo Y, Marcato P, Blandino G, Pass HI, Cioce M. Canino C, et al. Oncotarget. 2015 May 20;6(14):12637-53. doi: 10.18632/oncotarget.3703. Oncotarget. 2015. PMID: 25868979 Free PMC article. - CD44 and its ligand hyaluronan as potential biomarkers in malignant pleural mesothelioma: evidence and perspectives.
Cortes-Dericks L, Schmid RA. Cortes-Dericks L, et al. Respir Res. 2017 Apr 12;18(1):58. doi: 10.1186/s12931-017-0546-5. Respir Res. 2017. PMID: 28403901 Free PMC article. Review. - Activation of Matrix Hyaluronan-Mediated CD44 Signaling, Epigenetic Regulation and Chemoresistance in Head and Neck Cancer Stem Cells.
Bourguignon LYW, Earle C, Shiina M. Bourguignon LYW, et al. Int J Mol Sci. 2017 Aug 24;18(9):1849. doi: 10.3390/ijms18091849. Int J Mol Sci. 2017. PMID: 28837080 Free PMC article. Review.
Cited by
- Structure-Based Optimization of a Novel Class of Aldehyde Dehydrogenase 1A (ALDH1A) Subfamily-Selective Inhibitors as Potential Adjuncts to Ovarian Cancer Chemotherapy.
Huddle BC, Grimley E, Buchman CD, Chtcherbinine M, Debnath B, Mehta P, Yang K, Morgan CA, Li S, Felton J, Sun D, Mehta G, Neamati N, Buckanovich RJ, Hurley TD, Larsen SD. Huddle BC, et al. J Med Chem. 2018 Oct 11;61(19):8754-8773. doi: 10.1021/acs.jmedchem.8b00930. Epub 2018 Sep 28. J Med Chem. 2018. PMID: 30221940 Free PMC article. - Development of substituted benzimidazoles as inhibitors of human aldehyde dehydrogenase 1A isoenzymes.
Takahashi C, Chtcherbinine M, Huddle BC, Wilson MW, Emmel T, Hohlman RM, McGonigal S, Buckanovich RJ, Larsen SD, Hurley TD. Takahashi C, et al. Chem Biol Interact. 2024 Mar 1;391:110910. doi: 10.1016/j.cbi.2024.110910. Epub 2024 Feb 15. Chem Biol Interact. 2024. PMID: 38364885 Free PMC article. - Hypoxia promotes acquisition of aggressive phenotypes in human malignant mesothelioma.
Kim MC, Hwang SH, Kim NY, Lee HS, Ji S, Yang Y, Kim Y. Kim MC, et al. BMC Cancer. 2018 Aug 15;18(1):819. doi: 10.1186/s12885-018-4720-z. BMC Cancer. 2018. PMID: 30111297 Free PMC article. - Human Aldehyde Dehydrogenases: A Superfamily of Similar Yet Different Proteins Highly Related to Cancer.
Xanthis V, Mantso T, Dimtsi A, Pappa A, Fadouloglou VE. Xanthis V, et al. Cancers (Basel). 2023 Sep 4;15(17):4419. doi: 10.3390/cancers15174419. Cancers (Basel). 2023. PMID: 37686694 Free PMC article. Review. - A Pan-ALDH1A Inhibitor Induces Necroptosis in Ovarian Cancer Stem-like Cells.
Chefetz I, Grimley E, Yang K, Hong L, Vinogradova EV, Suciu R, Kovalenko I, Karnak D, Morgan CA, Chtcherbinine M, Buchman C, Huddle B, Barraza S, Morgan M, Bernstein KA, Yoon E, Lombard DB, Bild A, Mehta G, Romero I, Chiang CY, Landen C, Cravatt B, Hurley TD, Larsen SD, Buckanovich RJ. Chefetz I, et al. Cell Rep. 2019 Mar 12;26(11):3061-3075.e6. doi: 10.1016/j.celrep.2019.02.032. Cell Rep. 2019. PMID: 30865894 Free PMC article.
References
- Vandermeers F, Hubert P, Delvenne P, Mascaux C, Grigoriu B, Burny A, Scherpereel A, Willems L. Valproate, in combination with pemetrexed and cisplatin, provides additional efficacy to the treatment of malignant mesothelioma. Clin Cancer Res. 2009;15:2818–2828. doi: 10.1158/1078-0432.CCR-08-1579. - DOI - PubMed
- Koppaka V, Thompson DC, Chen Y, Ellermann M, Nicolaou KC, Juvonen RO, Petersen D, Deitrich RA, Hurley TD, Vasiliou V. Aldehyde dehydrogenase inhibitors: a comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol Rev. 2012;64:520–539. doi: 10.1124/pr.111.005538. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous