Synergistic effects of IL-4 and TNFα on the induction of B7-H1 in renal cell carcinoma cells inhibiting allogeneic T cell proliferation - PubMed (original) (raw)
Synergistic effects of IL-4 and TNFα on the induction of B7-H1 in renal cell carcinoma cells inhibiting allogeneic T cell proliferation
Dagmar Quandt et al. J Transl Med. 2014.
Abstract
Background: The importance of B7-H molecules for the T cell/tumor communication and its impact on renal cell carcinoma (RCC) progression and prognosis has been recently described. Cytokine treatment of RCC has earlier been shown to be beneficial in preclinical settings, but its clinical implementation has not proven to be as effective. This might be partially explained by the yet incomplete picture of cellular alterations in tumor cells upon cytokine treatment investigated in detail in this study.
Methods: RCC tumor cell lines were treated with different cytokines alone or in combination. The constitutive and/or cytokine-induced expression of cytokine receptors signaling components and B7-H molecules in RCC cells were analysed by qPCR and flow cytometry. A mcherry reporter gene construct containing B7-H1 promoter was cloned and its activity was determined upon transfection in cytokine-stimulated cells. Cytokine pretreated tumor cells were co-cultured with allogeneic CD8+ T cells from healthy donors and T cell proliferation as well as cytokine secretion was determined.
Results: A heterogeneous, but constitutive B7-H1,-H2,-H3 and H4 expression was found on human RCC cell lines. IL-4 and TNFα treatment led to strong synergistic induction of B7-H1 in RCC cells, whereas B7-H2 was only increased by TNFα. In contrast, B7-H3 and B7-H4 expression were not altered by these cytokines. Treatment of RCC cells with TNFα and IL-4 was accompanied by an activation of signaling molecules like NF-κB, IκB and STAT6. The cytokine-mediated up-regulation of B7-H1 was due to transcriptional control as determined by an increased B7-H1 promoter activity in the presence of IL-4 and TNFα. Despite HLA class I and LFA-1 were also increased, the cytokine-mediated up-regulation of B7-H1 was more pronounced and caused an inhibition of allospecifc CD8+ T cell proliferation.
Conclusion: Thus, IL-4 and TNFα, which could be released by immune cells of the tumor microenvironment, are able to control the B7-H1 expression in RCC thereby altering T cell responses. These data are of importance for understanding the complex interplay of tumor cells with immune cells orchestrated by a number of different soluble and membrane bound mediators and for the implementation of check point antibodies directed against B7-H1.
Figures
Figure 1
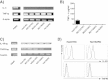
Expression analysis of IL-4, TNFα and their receptors in RCC cell lines. A) Constitutive TNFα mRNA expression, but lack of IL-4 mRNA expression was determined by conventional qPCR in different RCC cell lines (Hal31RC, MZ1257RC, MZ2514RC, MZ1790RC, Hal161RC) and in PBMC, which served as positive control. Representative data of at least 3 different experiments are shown. B) TNFα secretion of different RCC cell lines TNFα secretion was determined in Hal31RC, MZ1257RC, MZ2514RC, MZ1790RC, Hal161RC using ELISA. Representative data out of at least 3 different experiments are shown. C) The IL-4Rα and TNFRI-α expression was determined by qPCR in the different RCC cell lines and in PBMC, which served as positive control. Representative data of at least 3 different experiments are shown. D) Flow cytometric analyses of IL-4R and TNFαRI demonstrated the constitutive expression of IL-4R and TNFRI on Hal31RC and Hal161RC. The results are expressed as histograms. Bold line: staining, thin line: control. TNFRI is stained with an anti-TNFRI-PE labeled antibody, bold line: staining, thin line: isotype control. Representative data of at least 3 different experiments are shown.
Figure 2
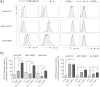
Significant increase of B7-H1 surface expression by IL-4 and TNFα treatment of RCC cell lines. Different RCC cell lines (Hal31RC, Hal161RC, MZ1790RC) were treated with either IL-4 or TNFα alone before B7-H expression was determined by flow cytometry as described in Materials and Methods at 72 hrs. A) Shown are FACS histogram overlay plots for B7-H1, B7-H2, B7-H3 and B7-H4. Each plot shows overlay graphs, depicting constitutive protein expression and expression upon single or combined cytokine treatment. Representative data of at least 3 different experiments are shown. B) Cytokine-mediated induction of B7-H1 and B7-H2 in % using constitutive expression levels as reference value. Combined data of 3 different experiments are given.
Figure 3
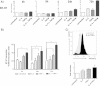
Time kinetic of B7-H1 expression and B7-H1 promoter targeting upon combined cytokine treatment with IL-4 and TNF α. A) Hal31RC was treated with either IL-4 or TNFα alone and with the combination of the two cytokines. B7-H1 protein expression was analyzed at the time points indicated by flow cytometry. Shown are fold increase in B7-H1 expression compared to untreated cells. Representative data of at least 3 different experiments are shown. B) B7-H1 mRNA expression in Hal31RC upon treatment with either IL-4 or TNFα alone and with the combination of the two cytokines for the time points indicated are shown. Using a B7-H1 plasmid as standard, B7-H1 transcript levels per 10.000 transcripts β-actin are shown. Data are obtained by real time PCR. Representative data of 3 different experiments are shown. C) BUF1088Mel was transfected with the mCherry B7-H1 promoter construct and stimulated with IL-4 and TNFα. The mCherry fluorescence was measured after 72 hrs by FACS and a representative histogram plot is depicted. The bar graph shows the combined result of a total of 3 independent assays.
Figure 4
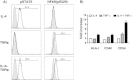
Involvement of signaling components and change of HLA-I, CD40 and CD54 expression upon combined cytokine treatment with IL-4 and TNF α in RCC cell lines. Hal31RC was treated with either IL-4 or TNFα alone and with the combination of the two cytokines. A) Intracellular pSTAT6 as well as NFκB (pS529) at 30 min post stimulation were analyzed by flow cytometry. Gray filled graph represents constitutive expression and black bold line represents cytokine-induced expression of either signaling component. Numbers on gates are percentage of positive cells compared to medium control. Representative data of at least 5 different experiments are shown. B) HLA class I, CD40, CD54 expression 72 hrs post cytokine treatment were analyzed by flow cytometry. Data are presented as fold increase when compared to constitutive expression of these molecules on untreated Hal31RC. Data are mean ± SEM of 9 different experiments.
Figure 5
Block of allogeneic CD8 + T cell proliferation upon coculture with RCC cells pretreated with IL-4 and TNFα . A-C) Hal31RC cells were treated with either IL-4 or TNFα alone and with the combination of both cytokines. CD8+ T cells from allogeneic PBMCs were sorted, CFDA-SE labeled and cocultured with cytokine pretreated RCC31Hal. A) Representative FACS histogramm plots of proliferated CD8+ Tcells cocultured with untreated (u RCC) as compared to IL-4 or TNFα pretreated Hal31RC (4 + T RCC) are shown. The division index (DI) is given. B) Summary of CD8+ T cell proliferation on cytokine pretreated Hal31RC. The division index (DI) is given. Pan anti-HLA class I antibody (w6/32) served as specificity control. Data are means of 4 different donors ± SEM. C) Partial reversal of T cell proliferation inhibition upon coculture of CD8+ T cells with anti-B7-H1 antibody or respective isotype control preincubated Hal31RC. Hal31RC were pretreated with IL-4 and TNFα for 3 days. Data represent the mean of 3 different donors ± SEM. D) Analyses of constitutive B7H ligand expression, ICOS, CD80 and PD-1 on allogeneic CD8+ T cells prior to coculture with RCC31. Data represent means of 7 different donors ± SEM. E) IFNγ production of isolated CD8+ T cells upon coculture of cytokine pretreated Hal31RC cells. Data are means of 3 different donors ± SEM.
Similar articles
- Gene transfer of the Co-stimulatory molecules B7-1 and B7-2 enhances the immunogenicity of human renal cell carcinoma to a different extent.
Jung D, Hilmes C, Knuth A, Jaeger E, Huber C, Seliger B. Jung D, et al. Scand J Immunol. 1999 Sep;50(3):242-9. doi: 10.1046/j.1365-3083.1999.00588.x. Scand J Immunol. 1999. PMID: 10447932 - IL-4 inhibits the TNF-alpha induced proliferation of renal cell carcinoma (RCC) and cooperates with TNF-alpha to induce apoptotic and cytokine responses by RCC: implications for antitumor immune responses.
Falkensammer C, Jöhrer K, Gander H, Ramoner R, Putz T, Rahm A, Greil R, Bartsch G, Thurnher M. Falkensammer C, et al. Cancer Immunol Immunother. 2006 Oct;55(10):1228-37. doi: 10.1007/s00262-006-0122-1. Epub 2006 Jan 28. Cancer Immunol Immunother. 2006. PMID: 16810557 Free PMC article. - Growth and major histocompatibility antigen expression regulation by IL-4, interferon-gamma (IFN-gamma) and tumour necrosis factor-alpha (TNF-alpha) on human renal cell carcinoma.
Hillman GG, Puri RK, Kukuruga MA, Pontes JE, Haas GP. Hillman GG, et al. Clin Exp Immunol. 1994 Jun;96(3):476-83. doi: 10.1111/j.1365-2249.1994.tb06054.x. Clin Exp Immunol. 1994. PMID: 8004818 Free PMC article. - Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy.
Thompson RH, Dong H, Kwon ED. Thompson RH, et al. Clin Cancer Res. 2007 Jan 15;13(2 Pt 2):709s-715s. doi: 10.1158/1078-0432.CCR-06-1868. Clin Cancer Res. 2007. PMID: 17255298 Review. - Significance of B7-H1 overexpression in kidney cancer.
Thompson RH, Kwon ED. Thompson RH, et al. Clin Genitourin Cancer. 2006 Dec;5(3):206-11. doi: 10.3816/CGC.2006.n.038. Clin Genitourin Cancer. 2006. PMID: 17239274 Review.
Cited by
- Increased antitumor efficacy of ginsenoside Rh2 via mixed micelles: in vivo and in vitro evaluation.
Xia X, Tao J, Ji Z, Long C, Hu Y, Zhao Z. Xia X, et al. Drug Deliv. 2020 Dec;27(1):1369-1377. doi: 10.1080/10717544.2020.1825542. Drug Deliv. 2020. PMID: 32998576 Free PMC article. - Autophagy controls programmed death-ligand 1 expression on cancer cells (Review).
Gao L, Chen Y. Gao L, et al. Biomed Rep. 2021 Oct;15(4):84. doi: 10.3892/br.2021.1460. Epub 2021 Aug 13. Biomed Rep. 2021. PMID: 34512972 Free PMC article. Review. - Prognostic value of PD-L1 overexpression for pancreatic cancer: evidence from a meta-analysis.
Zhuan-Sun Y, Huang F, Feng M, Zhao X, Chen W, Zhu Z, Zhang S. Zhuan-Sun Y, et al. Onco Targets Ther. 2017 Oct 16;10:5005-5012. doi: 10.2147/OTT.S146383. eCollection 2017. Onco Targets Ther. 2017. PMID: 29081663 Free PMC article. - Assessment of hazard immune-related genes and tumor immune infiltrations in renal cell carcinoma.
Chen H, Xie J, Jin P. Chen H, et al. Am J Transl Res. 2020 Nov 15;12(11):7096-7113. eCollection 2020. Am J Transl Res. 2020. PMID: 33312353 Free PMC article. - Emerging phagocytosis checkpoints in cancer immunotherapy.
Liu Y, Wang Y, Yang Y, Weng L, Wu Q, Zhang J, Zhao P, Fang L, Shi Y, Wang P. Liu Y, et al. Signal Transduct Target Ther. 2023 Mar 7;8(1):104. doi: 10.1038/s41392-023-01365-z. Signal Transduct Target Ther. 2023. PMID: 36882399 Free PMC article. Review.
References
- Organization WH: mortality database. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx 07/05/2013.
- Escudier B. Emerging immunotherapies for renal cell carcinoma. Ann Oncol. 2012;23(8):35–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous