VAPB/ALS8 interacts with FFAT-like proteins including the p97 cofactor FAF1 and the ASNA1 ATPase - PubMed (original) (raw)
VAPB/ALS8 interacts with FFAT-like proteins including the p97 cofactor FAF1 and the ASNA1 ATPase
Yorann Baron et al. BMC Biol. 2014.
Abstract
Background: FAF1 is a ubiquitin-binding adaptor for the p97 ATPase and belongs to the UBA-UBX family of p97 cofactors. p97 converts the energy derived from ATP hydrolysis into conformational changes of the p97 hexamer, which allows the dissociation of its targets from cellular structures or from larger protein complexes to facilitate their ubiquitin-dependent degradation. VAPB and the related protein VAPA form homo- and heterodimers that are anchored in the endoplasmic reticulum membrane and can interact with protein partners carrying a FFAT motif. Mutations in either VAPB or p97 can cause amyotrophic lateral sclerosis, a neurodegenerative disorder that affects upper and lower motor neurons.
Results: We show that FAF1 contains a non-canonical FFAT motif that allows it to interact directly with the MSP domain of VAPB and, thereby, to mediate VAPB interaction with p97. This finding establishes a link between two proteins that can cause amyotrophic lateral sclerosis when mutated, VAPB/ALS8 and p97/ALS14. Subsequently, we identified a similar FFAT-like motif in the ASNA1 subunit of the transmembrane-domain recognition complex (TRC), which in turn mediates ASNA1 interaction with the MSP domain of VAPB. Proteasome inhibition leads to the accumulation of ubiquitinated species in VAPB immunoprecipitates and this correlates with an increase in FAF1 and p97 binding. We found that VAPB interaction with ubiquitinated proteins is strongly reduced in cells treated with FAF1 siRNA. Our efforts to determine the identity of the ubiquitinated targets common to VAPB and FAF1 led to the identification of RPN2, a subunit of an oligosaccharyl-transferase located at the endoplasmic reticulum, which may be regulated by ubiquitin-mediated degradation.
Conclusions: The FFAT-like motifs we identified in FAF1 and ASNA1 demonstrate that sequences containing a single phenylalanine residue with the consensus (D/E)(D/E)FEDAx(D/E) are also proficient to mediate interaction with VAPB. Our findings indicate that the repertoire of VAPB interactors is more diverse than previously anticipated and link VAPB to the function of ATPase complexes such as p97/FAF1 and ASNA1/TRC.
Figures
Figure 1
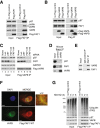
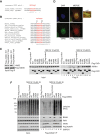
FAF1 interacts with VAPA and VAPB. (A) Flag-FAF1 immunoprecipitated from U2OS cells interacts with p97, VAPA and VAPB. (B) Flag-VAPA/B immunoprecipitated from U2OS cells interact with p97 and FAF1. (C) VAPB interaction with p97 is dependent on FAF1. U2OS cells were treated with the indicated siRNA oligos; luciferase (Luc) siRNA was used as a control. Flag-VAPB was immunoprecipitated and immunoblots of the immunoprecipitates (right) show that FAF1 depletion reduces the interaction with p97 whereas p97 depletion does not significantly affect the interaction with FAF1. (D) Endogenous VAPB interacts with p97 and FAF1 in mouse brain. Endogenous VAPB was immunoprecipitated from mouse brain extracts using Protein A-Sepharose (PAS) beads cross-linked to anti-VAPB antibodies. Uncoupled beads were used as a control. (E) Endogenous FAF1 interacts with VAPB in U2OS cells. The immunoprecipitation was performed using sheep anti-FAF1 antibody or sheep immunoglobulin G (IgG) as a control and PAS beads. (F) Indirect immunofluorescence of VAPB and wild-type (WT) Flag-FAF1. U2OS cells expressing Flag-FAF1 from a tetracycline-inducible promoter were grown in the presence of 200 ng/ml tetracycline for 24 hr and treated with 10 μM MG132 for 2 hr. Flag-FAF1 WT (red) co-localizes with VAPB (green) in a peri-nuclear area (enlarged window), suggesting an ER pattern. Scale bar is 10 μm. (G) VAPB levels and its interaction with Flag-FAF1 are not affected upon proteasome inhibition. Flag-FAF1 was immunoprecipitated from U2OS cells treated with 10 μM MG132 for 2 hr, 5 μM MG132 for 6 hr or left untreated (0 hr). Ubiquitinated proteins, p97 and VAPB were detected by immunoblotting in inputs (left) and immunoprecipitates (right). DAPI, 4',6-diamidino-2-phenylindole; IgG, immunoglobulin G; IP, immunoprecipitate; Luc, luciferase; WT, wild type.
Figure 2
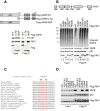
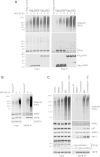
A FFAT-like motif mediates FAF1 interaction with VAPB. (A) The MSP domain of VAPB mediates its interaction with FAF1. Top panel: Schematic representation of the Flag-VAPB constructs, full length (1 to 243) or truncated, C-terminal half (125 to 243) and the MSP domain N-terminal half (1 to 125), immunoprecipitated from U2OS cells as shown in the bottom panel. Immunoblots of inputs (left) and immunoprecipitates (right) show that the MSP of VAPB is sufficient for the interaction with FAF1/p97. A highly conserved FFAT-like motif in FAF1 mediates its interaction with VAPB. (B) Top panel: Schematic representation of human FAF1 highlighting its various domains. Bottom panel: Wild-type or mutant variants of Flag-FAF1 were immunoprecipitated from U2OS cells. The indicated proteins were detected using specific antibodies in the inputs (left) and immunoprecipitates (right). UBA deletion caused a dramatic reduction in ubiquitinated protein binding to FAF1 whereas a point mutation in the UBX domain (P620G) abolished p97 binding. Deletion of the residues 293 to 301 was the only truncation that prevented VAPB binding to FAF1. (C) Alignment of FAF1 sequence from various species showing that the FFAT-like motif is highly conserved. (D) As in (B), showing that either a triple mutation D295A/F296A/E297A or single F296A mutation in FAF1 abolished VAPB binding. IP, immunoprecipitate; WT, wild type.
Figure 3
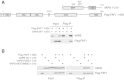
The P56S ALS-causing mutation of VAPB does not affect its interaction with FAF1 in vitro . The indicated variants of recombinant VAPB and Flag-FAF1 were incubated in vitro either alone or in combination, and then immunoprecipitated using anti-Flag beads. (A) Flag-FAF1 interacts directly with VAPB. Top panel: Schematic representation of the truncated version of VAPB (residues 1 to 210), lacking the C-terminal transmembrane region, and Flag-FAF1 full-length proteins expressed in bacteria. Bottom panel: Immunoblots of the indicated proteins in input extracts and anti-Flag immunoprecipitates. (B) The wild-type and the P56S mutant of VAPB (1 to 210), but not the K87D M89D double mutant, interact with Flag-FAF1 (right panel). IP, immunoprecipitate; WT, wild type.
Figure 4
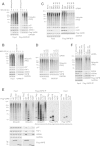
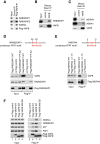
VAPB interaction with FAF1 and p97 is stimulated upon proteasome inhibition. (A) Proteasome inhibition enhances ubiquitin, p97 and FAF1 binding to Flag-VAPB, while ASNA1 binding remains largely unchanged. U2OS cells expressing Flag-VAPB from a tetracycline-inducible promoter were grown in the presence of 100 ng/ml tetracycline for 24 hr or left untreated as a control. Flag-VAPB was immunoprecipitated using anti-Flag beads. (B) Proteasome inhibition enhances ubiquitin, p97 and FAF1 binding to endogenous VAPB, while ASNA1 binding was only slightly increased. Endogenous VAPB was immunoprecipitated from U2OS cells using anti-VAPB antibodies cross-linked to Protein A-beads. Uncoupled beads were used as control. (C, D) The binding of ubiquitinated proteins to VAPB is largely mediated by FAF1. U2OS cells were treated with the indicated siRNA oligos. Flag-VAPB (C) or endogenous VAPB (D) were immunoprecipitated as described above. Depletion of FAF1 strongly reduces the binding of ubiquitinated proteins to Flag- or endogenous VAPB. (E) The K87D M89D double mutant (KM-DD) of VAPB is defective in p97, FAF1, ASNA1 and ubiquitin binding. Flag-VAPB full-length WT, KM-DD and truncated, C-terminal half (C-Ter) and N-terminal half (MSP), were immunoprecipitated from U2OS cells. The MSP domain of VAPB is sufficient to interact with p97, FAF1 and ASNA1. KM-DD as well as the truncation lacking the MSP domain (C-Ter) are defective in binding poly-ubiquitinated proteins and seem to interact preferentially with oligo-ubiquitinated proteins. (F) The binding of ubiquitinated proteins to VAPB is reduced in cells treated with ASNA1 siRNA. U2OS cells expressing Flag-VAPB from a tetracycline-inducible promoter were treated with the indicated siRNA oligos. Depletion of ASNA1 reduces ubiquitin and BAG6 binding, but not FAF1 binding, to Flag-VAPB. C-Ter, C-terminal half; IP, immunoprecipitate; KM-DD, K87D M89D double mutant; Luc, luciferase.
Figure 5
ASNA1 interacts with VAPB via a FFAT-like motif similar to FAF1. (A) Alignment of the FFAT-like motifs in human ASNA1 and FAF1. (B) Alignment of the FFAT-like motif of ASNA1 across species showing that it is highly conserved. (C) A point mutation in the FFAT-like motif of ASNA1 (F15A) abolishes its interaction with VAPB. WT and mutant Flag-ASNA1 were immunoprecipitated from U2OS cells using anti-Flag beads. (D) Indirect immunofluorescence of VAPB and Flag-ASNA1 WT. U2OS cells were transfected with Flag-ASNA1 WT for 24 hr. Flag-ASNA1 WT (red) is co-localized with VAPB (green) in a peri-nuclear area (enlarged window) suggesting an ER pattern. Scale bar is 10 μm. (E) ASNA1 interaction with FAF1 is strongly stimulated upon proteasome inhibition with MG132 and depends on the UBA domain. WT Flag-FAF1 and the indicated mutants were immunoprecipitated from U2OS cells. (F) G46R and G46A point mutations in ASNA1 abolish its interaction with BAG6 and strongly reduce its interaction with FAF1 and ubiquitin, most noticeably after MG132 treatment, but do not affect the interaction with VAPB. WT and mutant Flag-ASNA1 were immunoprecipitated from SH-SY5Y cells. DAPI, 4',6-diamidino-2-phenylindole; IP, immunoprecipitate; WT, wild type.
Figure 6
RPN2 is a common interactor of VAPB and FAF1. (A) Flag-VAPB and Flag-FAF1 were immunoprecipitated from U2OS cells treated with 10 μM MG132 for 2 hr, 5 μM MG132 for 6 hr or left untreated (0 hr). Asterisks indicate non-specific bands. (B) Proteasome inhibition enhances RPN2 binding to endogenous VAPB. Endogenous VAPB was immunoprecipitated from U2OS cells treated with 10 μM MG132 for 2 hr or left untreated (0 hr). (C) ER stress induced by tunicamycin treatment does not affect RPN2 levels or its binding to VAPB. Endogenous VAPB was immunoprecipitated from U2OS cells treated with 5 μg/ml tunicamycin for 5 h, 10 μM MG132 for 2 hr, both tunicamycin and MG132, or dimethyl sulfoxide as a control. DMSO, dimethyl sulfoxide; IP, immunoprecipitate; MG, MG132.
Figure 7
RAB3GAP1 and WDR44 are FFAT-like interactors of VAPB. (A) Flag-VAPB immunoprecipitated from HeLa cells interacts with RAB3GAP1, RAB3GAP2 and WDR44. The RAB3GAP1 and WDR44 interactions with VAPB are mediated by FFAT motifs. VAPB immunoprecipitation from mouse brain extract confirms its interaction with RAB3GAP1 (B), ASNA1 and WDR44 (C). Flag-RAB3GAP1 WT and F585A/F586A (FF-AA) (D) and Flag-WDR44 WT and Δ1-15 (E) were immunoprecipitated from U2OS cells. Both RAB3GAP1 and WDR44 contain FFAT motifs, as indicated in the top panels, which are responsible for the interaction with VAPB. The formation of the RAB3GAP1/2 heterodimer is not affected by the mutation of the FFAT motif in RAB3GAP1. (F) Immunoprecipitation of Flag-VAPA WT, Flag-VAPB WT or the K87D M89D mutant of VAPB from U2OS cells shows that the KM-DD mutant is defective in interacting with RAB3GAP1 and WDR44. FF-AA, F585A/F586A; IP, immunoprecipitate.
Figure 8
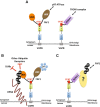
VAPB interacts with novel FFAT-like proteins, FAF1 and ASNA1. (A) The FFAT-like motif of FAF1 mediates its interaction with the MSP domain of VAPB and recruits FAF1/p97 to the ER membrane. Similarly, the FFAT-like sequence in ASNA1 mediates VAPB interaction with the TRC complex. (B) RPN2 is a common ubiquitinated target for FAF1 and VAPB. We propose that, on one side, FAF1 protects ubiquitinated RPN2 from deubiquitinating enzymes (DUBs) and other ubiquitin receptors and, on the other side, it recruits p97 hexamers that are necessary for extracting misfolded or misassembled RPN2 from the ER membrane, to allow for its proteasome-mediated degradation. (C) ASNA1 mediates VAPB interaction with a subset of ubiquitinated targets that interact with FAF1 via their ubiquitin chains. We propose that VAPB might represent an alternative receptor for TRCs that are implicated in clearing mislocalized ER proteins.
Similar articles
- Caspar, an adapter for VAPB and TER94, modulates the progression of ALS8 by regulating IMD/NFκB-mediated glial inflammation in a Drosophila model of human disease.
Tendulkar S, Hegde S, Garg L, Thulasidharan A, Kaduskar B, Ratnaparkhi A, Ratnaparkhi GS. Tendulkar S, et al. Hum Mol Genet. 2022 Aug 25;31(17):2857-2875. doi: 10.1093/hmg/ddac076. Hum Mol Genet. 2022. PMID: 35377453 Free PMC article. - Complex of Fas-associated factor 1 (FAF1) with valosin-containing protein (VCP)-Npl4-Ufd1 and polyubiquitinated proteins promotes endoplasmic reticulum-associated degradation (ERAD).
Lee JJ, Park JK, Jeong J, Jeon H, Yoon JB, Kim EE, Lee KJ. Lee JJ, et al. J Biol Chem. 2013 Mar 8;288(10):6998-7011. doi: 10.1074/jbc.M112.417576. Epub 2013 Jan 4. J Biol Chem. 2013. PMID: 23293021 Free PMC article. - Crystallization and preliminary X-ray crystallographic analysis of the N domain of p97/VCP in complex with the UBX domain of FAF1.
Shin HY, Kang W, Lee SY, Yang JK. Shin HY, et al. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010 Jan 1;66(Pt 1):41-3. doi: 10.1107/S1744309109047691. Epub 2009 Dec 25. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010. PMID: 20057067 Free PMC article. - Insights into adaptor binding to the AAA protein p97.
Yeung HO, Kloppsteck P, Niwa H, Isaacson RL, Matthews S, Zhang X, Freemont PS. Yeung HO, et al. Biochem Soc Trans. 2008 Feb;36(Pt 1):62-7. doi: 10.1042/BST0360062. Biochem Soc Trans. 2008. PMID: 18208387 Review. - The Link between VAPB Loss of Function and Amyotrophic Lateral Sclerosis.
Borgese N, Iacomino N, Colombo SF, Navone F. Borgese N, et al. Cells. 2021 Jul 23;10(8):1865. doi: 10.3390/cells10081865. Cells. 2021. PMID: 34440634 Free PMC article. Review.
Cited by
- Phenotypic heterogeneity in amyotrophic lateral sclerosis type 8 and modifying mechanisms of neurodegeneration.
Oliveira D, Verjovski-Almeida S, Zatz M. Oliveira D, et al. Neural Regen Res. 2021 Sep;16(9):1776-1778. doi: 10.4103/1673-5374.303030. Neural Regen Res. 2021. PMID: 33510073 Free PMC article. No abstract available. - Caspar, an adapter for VAPB and TER94, modulates the progression of ALS8 by regulating IMD/NFκB-mediated glial inflammation in a Drosophila model of human disease.
Tendulkar S, Hegde S, Garg L, Thulasidharan A, Kaduskar B, Ratnaparkhi A, Ratnaparkhi GS. Tendulkar S, et al. Hum Mol Genet. 2022 Aug 25;31(17):2857-2875. doi: 10.1093/hmg/ddac076. Hum Mol Genet. 2022. PMID: 35377453 Free PMC article. - The Interactome of the VAP Family of Proteins: An Overview.
James C, Kehlenbach RH. James C, et al. Cells. 2021 Jul 14;10(7):1780. doi: 10.3390/cells10071780. Cells. 2021. PMID: 34359948 Free PMC article. Review. - Lgl reduces endosomal vesicle acidification and Notch signaling by promoting the interaction between Vap33 and the V-ATPase complex.
Portela M, Yang L, Paul S, Li X, Veraksa A, Parsons LM, Richardson HE. Portela M, et al. Sci Signal. 2018 Jun 5;11(533):eaar1976. doi: 10.1126/scisignal.aar1976. Sci Signal. 2018. PMID: 29871910 Free PMC article. - Proteomics-Based Approach Identifies Altered ER Domain Properties by ALS-Linked VAPB Mutation.
Yamanaka T, Nishiyama R, Shimogori T, Nukina N. Yamanaka T, et al. Sci Rep. 2020 May 6;10(1):7610. doi: 10.1038/s41598-020-64517-z. Sci Rep. 2020. PMID: 32376919 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials