p-Tau immunotherapy reduces soluble and insoluble tau in aged 3xTg-AD mice - PubMed (original) (raw)
p-Tau immunotherapy reduces soluble and insoluble tau in aged 3xTg-AD mice
Ken C Walls et al. Neurosci Lett. 2014.
Abstract
Alzheimer's disease (AD) is a proteinopathy characterized by the accumulation of β-amyloid (Aβ) and tau. To date, clinical trials indicate that Aβ immunotherapy does not improve cognition. Consequently, it is critical to modulate other aspects of AD pathology. As such, tau represents an excellent target, as its accumulation better correlates with cognitive impairment. To determine the effectiveness of targeting pathological tau, with Aβ pathology present, we administered a single injection of AT8, or control antibody, into the hippocampus of aged 3xTg-AD mice. Extensive data indicates that phosphorylated Ser(202) and Thr(205) sites of tau (corresponding to the AT8 epitope) represent a pathologically relevant target for AD. We report that immunization with AT8 reduced somatodendritic tau load, p-tau immunoreactivity, and silver stained positive neurons, without affecting Aβ pathology. We also discovered that tau pathology soon reemerges post-injection, possibly due to persistent Aβ pathology. These studies provide evidence that targeting p-tau may represent an effective treatment strategy: potentially in conjunction with Aβ immunotherapy.
Keywords: Alzheimer's disease; Beta-amyloid; Immunotherapy; Neurofibrillary tangles; Phosphorylated tau; Tau.
Copyright © 2014. Published by Elsevier Ireland Ltd.
Figures
Fig. 1
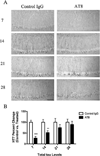
Immunization with the AT8-antibody reduces somatodendritic tau immunoreactivity. Total tau levels were quantified in 15–18-month-old 3xTg-AD mice following a single intrahippocampal injection with AT8 or a control IgG. Mice were sacrificed at post injection day 7, 14, 21, or 28. (A) Immunohistochemical analysis reveled sharp decreases in total tau (HT7) levels after immunization with AT8 at days 7 and 14, but not days 21 and 28. (B) Statistical analysis of the change in total tau between AT8 treated and control IgG treated hippocampal sides (_t_-test, ***P < 0.0001, **P < 0.008, N = 3–7). Scale bar equals 500 µm.
Fig. 2
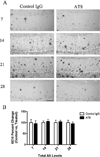
Tau immunization does not affect Aβ pathology. Total Aβ levels were also quantified following AT8 injection at post injection day 7, 14, 21, or 28. (A) In contrast to what was observed for tau, immunohistochemical analysis against Aβ (6E10) detected no differences between AT8 and control IgG treated hippocampi at either time point analyzed. (B) Statistical analysis of the change in total Aβ load between AT8 treated and control IgG treated hippocampal sides (N = 3–7). Scale bar equals 500 µm.
Fig. 3
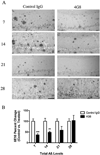
Immunization with the 4G8-antibody reduces intra- and extracellular Aβ. In addition to treatment with AT8, a small cohort of 15–18-month-old 3xTg-AD mice was treated with either the anti-Aβ antibody 4G8 or a control IgG. (A) Staining for total Aβ load (6E10) revealed that 4G8 injection drastically reduced intra- and extracellular Aβ at days 7 and 14. (B) Statistical analysis of Aβ immunization in 4G8 vs. control IgG hippocampal sections (_t_-test, ***P < 0.0003, **P < 0.0057, N = 3–7). Scale bar equals 500 µm.
Fig. 4
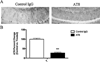
Targeting p-tau via immunization significantly reduces early AT8 immunoreactivity. The phosphorylated residues of tau that make up the AT8 epitope also represent some of the earliest modifications observed in tau pathogenesis. (A) Staining for phosphorylated residues Ser199 and Thr202 (AT8) show a large decreased in reactive at 7 days post-injection. (B) Statistical analysis of AT8 immunoreactivity, at post injection day 7, reveals a significant decrease in AT8 treated vs. control IgG treated sections (_t_-test, ***P < 0.0001, N = 7). Scale bar equals 250 µm.
Fig. 5
3xTg-AD mice immunized with AT8 have reduced Gallyas positive neurons one week post-injection. (A) Using the Gallyas silver stain method, we observed fewer Gallyas positive neurons in hemispheres treated with AT8 at 7 days. (B) Statistical analysis of Gallyas staining after AT8 treatment (_t_-test, ***P < 0.0001, N = 7). Scale bar equals 250 µm.
Similar articles
- Immunotherapy to improve cognition and reduce pathological species in an Alzheimer's disease mouse model.
Herline K, Prelli F, Mehta P, MacMurray C, Goñi F, Wisniewski T. Herline K, et al. Alzheimers Res Ther. 2018 Jun 18;10(1):54. doi: 10.1186/s13195-018-0384-9. Alzheimers Res Ther. 2018. PMID: 29914551 Free PMC article. - Tau passive immunization inhibits not only tau but also Aβ pathology.
Dai CL, Tung YC, Liu F, Gong CX, Iqbal K. Dai CL, et al. Alzheimers Res Ther. 2017 Jan 10;9(1):1. doi: 10.1186/s13195-016-0227-5. Alzheimers Res Ther. 2017. PMID: 28073379 Free PMC article. - Passive immunization targeting the N-terminal projection domain of tau decreases tau pathology and improves cognition in a transgenic mouse model of Alzheimer disease and tauopathies.
Dai CL, Chen X, Kazim SF, Liu F, Gong CX, Grundke-Iqbal I, Iqbal K. Dai CL, et al. J Neural Transm (Vienna). 2015 Apr;122(4):607-17. doi: 10.1007/s00702-014-1315-y. Epub 2014 Sep 19. J Neural Transm (Vienna). 2015. PMID: 25233799 - Immunotherapy for Alzheimer's disease.
Wang W, Fan L, Xu D, Wen Z, Yu R, Ma Q. Wang W, et al. Acta Biochim Biophys Sin (Shanghai). 2012 Oct;44(10):807-14. doi: 10.1093/abbs/gms065. Epub 2012 Aug 16. Acta Biochim Biophys Sin (Shanghai). 2012. PMID: 22899646 Review. - Tau-based therapeutic approaches for Alzheimer's disease - a mini-review.
Boutajangout A, Wisniewski T. Boutajangout A, et al. Gerontology. 2014;60(5):381-5. doi: 10.1159/000358875. Epub 2014 Apr 8. Gerontology. 2014. PMID: 24732638 Free PMC article. Review.
Cited by
- Human tau increases amyloid β plaque size but not amyloid β-mediated synapse loss in a novel mouse model of Alzheimer's disease.
Jackson RJ, Rudinskiy N, Herrmann AG, Croft S, Kim JM, Petrova V, Ramos-Rodriguez JJ, Pitstick R, Wegmann S, Garcia-Alloza M, Carlson GA, Hyman BT, Spires-Jones TL. Jackson RJ, et al. Eur J Neurosci. 2016 Dec;44(12):3056-3066. doi: 10.1111/ejn.13442. Epub 2016 Nov 12. Eur J Neurosci. 2016. PMID: 27748574 Free PMC article. - Current Status of Clinical Trials on Tau Immunotherapies.
Ji C, Sigurdsson EM. Ji C, et al. Drugs. 2021 Jul;81(10):1135-1152. doi: 10.1007/s40265-021-01546-6. Epub 2021 Jun 8. Drugs. 2021. PMID: 34101156 Free PMC article. Review. - Prophylactic Active Tau Immunization Leads to Sustained Reduction in Both Tau and Amyloid-β Pathologies in 3xTg Mice.
Rajamohamedsait H, Rasool S, Rajamohamedsait W, Lin Y, Sigurdsson EM. Rajamohamedsait H, et al. Sci Rep. 2017 Dec 6;7(1):17034. doi: 10.1038/s41598-017-17313-1. Sci Rep. 2017. PMID: 29213096 Free PMC article. - Tau immunotherapy modulates both pathological tau and upstream amyloid pathology in an Alzheimer's disease mouse model.
Castillo-Carranza DL, Guerrero-Muñoz MJ, Sengupta U, Hernandez C, Barrett AD, Dineley K, Kayed R. Castillo-Carranza DL, et al. J Neurosci. 2015 Mar 25;35(12):4857-68. doi: 10.1523/JNEUROSCI.4989-14.2015. J Neurosci. 2015. PMID: 25810517 Free PMC article. - Novel Phospho-Tau Monoclonal Antibody Generated Using a Liposomal Vaccine, with Enhanced Recognition of a Conformational Tauopathy Epitope.
Theunis C, Adolfsson O, Crespo-Biel N, Piorkowska K, Pihlgren M, Hickman DT, Gafner V, Borghgraef P, Devijver H, Pfeifer A, Van Leuven F, Muhs A. Theunis C, et al. J Alzheimers Dis. 2017;56(2):585-599. doi: 10.3233/JAD-160695. J Alzheimers Dis. 2017. PMID: 28035925 Free PMC article.
References
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N. Engl. J. Med. 2010;362:329–344. - PubMed
- Roberson ED2, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. - PubMed
- Dumanchin C, Camuzat A, Campion D, Verpillat P, Hannequin D, Dubois B, Saugier-Veber P, Martin C, Penet C, Charbonnier F, Agid Y, Frebourg T, Brice A. Segregation of a missense mutation in the microtubule-associated protein tau gene with familial frontotemporal dementia and parkinsonism. Hum. Mol. Genet. 1998;7:1825–1829. - PubMed
- Goedert M, Klug A, Crowther RA. Tau protein, the paired helical filament and Alzheimer’s disease. J. Alzheimers Dis. 2006;9:195–207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials