Sigma-1 receptor antagonism restores injury-induced decrease of voltage-gated Ca2+ current in sensory neurons - PubMed (original) (raw)
Sigma-1 receptor antagonism restores injury-induced decrease of voltage-gated Ca2+ current in sensory neurons
Bin Pan et al. J Pharmacol Exp Ther. 2014 Aug.
Abstract
Sigma-1 receptor (σ1R), an endoplasmic reticulum-chaperone protein, can modulate painful response after peripheral nerve injury. We have demonstrated that voltage-gated calcium current is inhibited in axotomized sensory neurons. We examined whether σ1R contributes to the sensory dysfunction of voltage-gated calcium channel (VGCC) after peripheral nerve injury through electrophysiological approach in dissociated rat dorsal root ganglion (DRG) neurons. Animals received either skin incision (Control) or spinal nerve ligation (SNL). Both σ1R agonists, (+)pentazocine (PTZ) and DTG [1,3-di-(2-tolyl)guanidine], dose dependently inhibited calcium current (ICa) with Ba(2+) as charge carrier in control sensory neurons. The inhibitory effect of σ1R agonists on ICa was blocked by σ1R antagonist, BD1063 (1-[2-(3,4-dichlorophenyl)ethyl]-4-methylpiperazine dihydrochloride) or BD1047 (N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine dihydrobromide). PTZ and DTG showed similar effect on ICa in axotomized fifth DRG neurons (SNL L5). Both PTZ and DTG shifted the voltage-dependent activation and steady-state inactivation of VGCC to the left and accelerated VGCC inactivation rate in both Control and axotomized L5 SNL DRG neurons. The σ1R antagonist, BD1063 (10 μM), increases ICa in SNL L5 neurons but had no effect on Control and noninjured fourth lumbar neurons in SNL rats. Together, the findings suggest that activation of σR1 decreases ICa in sensory neurons and may play a pivotal role in pain generation.
U.S. Government work not protected by U.S. copyright.
Figures
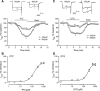
Fig. 1.
_σ_1R agonists inhibit voltage-gated calcium current in control sensory neurons. After whole-cell configuration, the _I_Ca was measured with a square wave voltage command (holding potential at −90 mV and step to 0 mV for 100 milliseconds) using Ba2+ as charge carrier. PTZ (A) reduced _I_Ca (sample traces, top panel; averaged time data, bottom panel) in a dose-related fashion (B), as did DTG (C and D). N = 4–10 for each concentration.
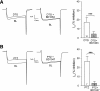
Fig. 2.
Effect of PTZ and DTG on _I_Ca is mediated by _σ_1R. After whole-cell configuration, the _I_Ca was measured with a square wave voltage command (holding potential at −100 mV and step to 0 mV for 50 milliseconds). Cells were preincubated with BD1047 or BD1063 for 15 minutes before PTZ and DTG administration. (A) Sample traces from control animal showed that DTG (10 _μ_M) inhibited _I_Ca and the inhibition was prevented by BD1063 (10 _μ_M). (B) Sample traces from control animal showed that PTZ (100 _μ_M) inhibited _I_Ca and the inhibition was prevented by BD1047 (10 _μ_M). Summary data of _I_Ca inhibition were derived from the current density, which was normalized to its baseline current density. Mean ± S.E.M.; number in bars represents the sample size; ***P < 0.001. BL, baseline.
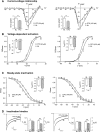
Fig. 3.
_σ_1R modulates kinetic properties of VGCC in control animals. (A) _I_Ca was measured with a square wave voltage command (200 milliseconds; 10 mV increment from −100 to 50 mV; holding potential at −65 mV). Average inward current density (pA/pF) against current-voltage relationship (I-V) elicited by PTZ (50 _μ_M) and DTG (100 _μ_M) was fit to a single Boltzmann function (see Materials and Methods). (B) Voltage-dependent activation of VGCC was derived from the I-V curve by Boltzmann analysis. (C) The steady-state inactivation of _I_Ca was measured during a 4-second square wave depolarization (−100 to 30 mV in 10-mV increments) with _I_Ca determined by a subsequent test pulse (20 milliseconds to 0 mV). Results are normalized to maximal peak current (I/_I_max). (D) A simple step protocol (−100 to 0 mV for 2 seconds) was applied to measure the inactivation kinetics by a two-exponential function (_τ_1 and _τ_2). Inset figures revealed summary data of maximal conductance (_G_max; A), voltage at which current is half-maximal (_V_1/2; B and C), and inactivation constant (τ; D). Mean ± S.E.M.; number in bars represents the sample size; *P < 0.05; **P < 0.01; ***P < 0.001. BL, baseline.
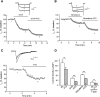
Fig. 4.
Inhibition of _σ_1R agonists on _I_Ca encompasses multiple _I_Ca subtypes in control sensory neurons. (A) In whole-cell configuration, _I_Ca was measured with a square wave voltage command (holding potential at −90 mV and step to 0 mV for 150 milliseconds). Sample current traces (top) and averaged data (bottom panel) show response to _σ_1R activation by PTZ (100 _µ_M) after blockade of N-type channels with _ω_-conotoxin GVIA (GVIA, 200 nM). (B) Similarly, sensitivity to PTZ is demonstrated after blockade of L-type channels with nimodipine (5 _µ_M). (C) T-type _I_Ca was triggered by depolarizations from a holding potential at −100 mV and step to −30 mV after incubation (20 minutes) with R-type current blocker SNX-482 (200 nM) and P/Q-type current blocker _ω_-conotoxin MVIIC (200 nM), and addition of L-type current blocker nimodipine (5 _µ_M) and GVIA (200 nM) to the recording bath. These currents also showed sensitivity to PTZ. (D) Summary data. Mean ± S.E.M.; number in bars represents the sample size; **P < 0.01; ***P < 0.001. BL, baseline.
Fig. 5.
_σ_1R modulates kinetics of VGCC in axotomized animals. After whole-cell configuration, the current-voltage relationship (I-V), voltage-dependent activation, and steady-state inactivation of the _I_Ca were measured as described in Fig. 4 using Ba2+ as charge carrier in SNL L5 DRG sensory neurons. (A) Average inward current density (pA/pF) against I-V relationship elicited by PTZ (50 _μ_M) and DTG (100 _μ_M) was fit to a single Boltzmann function (see Materials and Methods). (B) Voltage-dependent activation of VGCC was derived from the I-V curve. (C) In steady-state inactivation, _V_1/2 was shifted toward a hyperpolarization direction. (D) Sample traces showed that both PTZ and DTG administration decreased slow inactivation constant (_τ_2). Inset figures revealed summary data of maximal conductance (_G_max; A), voltage at which current is half-maximal (_V_1/2; B and C), and inactivation constant (τ; D). Mean ± S.E.M.; number in bars represents the sample size; *P < 0.05; **P < 0.01; ***P < 0.001. BL, baseline.
Fig. 6.
_σ_1R antagonist, BD1063 (10 _μ_M), increased _I_Ca in axotomized fifth (SNL L5) DRG sensory neurons. After whole-cell configuration, the _I_Ca was measured with a square wave voltage command (holding potential at −100 mV and step to 0 mV for 20 milliseconds) using Ba2+ as charge carrier. Sample current traces (A and B) showed that BD1063 (10 _μ_M) did not affect _I_Ca in control and noninjured neighboring fourth (SNL L4) DRG neurons. (C) BD1063 administration increased the _I_Ca in SNL L5 neurons. Summary data demonstrated that application of BD1063 (10 _μ_M) was able to increase in SNL L5 but not control and SNL L4 neurons. Mean ± S.E.M.; number in bars represents the sample size; **P < 0.01. BL, baseline.
Similar articles
- Sigma-1 receptor expression in sensory neurons and the effect of painful peripheral nerve injury.
Bangaru ML, Weihrauch D, Tang QB, Zoga V, Hogan Q, Wu HE. Bangaru ML, et al. Mol Pain. 2013 Sep 10;9:47. doi: 10.1186/1744-8069-9-47. Mol Pain. 2013. PMID: 24015960 Free PMC article. - Icilin reduces voltage-gated calcium channel currents in naïve and injured DRG neurons in the rat spinal nerve ligation model.
Hagenacker T, Lampe M, Schäfers M. Hagenacker T, et al. Brain Res. 2014 Apr 4;1557:171-9. doi: 10.1016/j.brainres.2014.02.022. Epub 2014 Feb 18. Brain Res. 2014. PMID: 24560602 - Upregulation of N-type calcium channels in the soma of uninjured dorsal root ganglion neurons contributes to neuropathic pain by increasing neuronal excitability following peripheral nerve injury.
Yang J, Xie MX, Hu L, Wang XF, Mai JZ, Li YY, Wu N, Zhang C, Li J, Pang RP, Liu XG. Yang J, et al. Brain Behav Immun. 2018 Jul;71:52-65. doi: 10.1016/j.bbi.2018.04.016. Epub 2018 Apr 27. Brain Behav Immun. 2018. PMID: 29709527 - Subtype-specific reduction of voltage-gated calcium current in medium-sized dorsal root ganglion neurons after painful peripheral nerve injury.
McCallum JB, Wu HE, Tang Q, Kwok WM, Hogan QH. McCallum JB, et al. Neuroscience. 2011 Apr 14;179:244-55. doi: 10.1016/j.neuroscience.2011.01.049. Epub 2011 Jan 28. Neuroscience. 2011. PMID: 21277351 Free PMC article. - Chaperone Sigma1R and Antidepressant Effect.
Voronin MV, Vakhitova YV, Seredenin SB. Voronin MV, et al. Int J Mol Sci. 2020 Sep 25;21(19):7088. doi: 10.3390/ijms21197088. Int J Mol Sci. 2020. PMID: 32992988 Free PMC article. Review.
Cited by
- Elevated Expression and Activity of Sodium Leak Channel Contributes to Neuronal Sensitization of Inflammatory Pain in Rats.
Li J, Chen Y, Liu J, Zhang D, Liang P, Lu P, Shen J, Miao C, Zuo Y, Zhou C. Li J, et al. Front Mol Neurosci. 2021 Aug 27;14:723395. doi: 10.3389/fnmol.2021.723395. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34512260 Free PMC article. - The Sigma-1 Receptor: When Adaptive Regulation of Cell Electrical Activity Contributes to Stimulant Addiction and Cancer.
Soriani O, Kourrich S. Soriani O, et al. Front Neurosci. 2019 Nov 12;13:1186. doi: 10.3389/fnins.2019.01186. eCollection 2019. Front Neurosci. 2019. PMID: 31780884 Free PMC article. Review. - A Practical Guide to Sigma-1 Receptor Positron Emission Tomography/Magnetic Resonance Imaging: A New Clinical Molecular Imaging Method to Identify Peripheral Pain Generators in Patients with Chronic Pain.
Shen B, Yoon D, Castillo J, Biswal S. Shen B, et al. Semin Musculoskelet Radiol. 2023 Dec;27(6):601-617. doi: 10.1055/s-0043-1775744. Epub 2023 Nov 7. Semin Musculoskelet Radiol. 2023. PMID: 37935207 Free PMC article. - The Sigma-1 Receptor as a Pluripotent Modulator in Living Systems.
Su TP, Su TC, Nakamura Y, Tsai SY. Su TP, et al. Trends Pharmacol Sci. 2016 Apr;37(4):262-278. doi: 10.1016/j.tips.2016.01.003. Epub 2016 Feb 9. Trends Pharmacol Sci. 2016. PMID: 26869505 Free PMC article. Review. - Sigma-1 receptor ligands inhibit catecholamine secretion from adrenal chromaffin cells due to block of nicotinic acetylcholine receptors.
Brindley RL, Bauer MB, Hartley ND, Horning KJ, Currie KPM. Brindley RL, et al. J Neurochem. 2017 Oct;143(2):171-182. doi: 10.1111/jnc.14149. Epub 2017 Sep 19. J Neurochem. 2017. PMID: 28815595 Free PMC article.
References
- Alonso G, Phan V, Guillemain I, Saunier M, Legrand A, Anoal M, Maurice T. (2000) Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience 97:155–170 - PubMed
- Auer S, Ibañez-Tallon I. (2010) “The King is dead”: checkmating ion channels with tethered toxins. Toxicon 56:1293–1298 - PubMed
- Berkefeld H, Fakler B, Schulte U. (2010) Ca2+-activated K+ channels: from protein complexes to function. Physiol Rev 90:1437–1459 - PubMed
- Bourinet E, Altier C, Hildebrand ME, Trang T, Salter MW, Zamponi GW. (2014) Calcium-permeable ion channels in pain signaling. Physiol Rev 94:81–140 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K01 DA024751/DA/NIDA NIH HHS/United States
- R01 NS042150/NS/NINDS NIH HHS/United States
- R01-NS42150/NS/NINDS NIH HHS/United States
- K01-DA024751/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous