Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease - PubMed (original) (raw)
Review
Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease
Peter C Harris et al. J Clin Invest. 2014 Jun.
Abstract
Recent advances in defining the genetic mechanisms of disease causation and modification in autosomal dominant polycystic kidney disease (ADPKD) have helped to explain some extreme disease manifestations and other phenotypic variability. Studies of the ADPKD proteins, polycystin-1 and -2, and the development and characterization of animal models that better mimic the human disease, have also helped us to understand pathogenesis and facilitated treatment evaluation. In addition, an improved understanding of aberrant downstream pathways in ADPKD, such as proliferation/secretion-related signaling, energy metabolism, and activated macrophages, in which cAMP and calcium changes may play a role, is leading to the identification of therapeutic targets. Finally, results from recent and ongoing preclinical and clinical trials are greatly improving the prospects for available, effective ADPKD treatments.
Figures
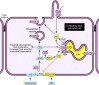
Figure 1. An abnormal crosstalk of calcium and cAMP signaling disrupts multiple signaling pathways and leads to the cystic phenotype.
Activation of calcium-inhibitable adenylyl cyclase 6 (AC6) and inhibition of calcium/calmodulin-dependent phosphodiesterase 1 (PDE1) causes abnormal accumulation of cAMP and activation of PKA. Disrupted intracellular calcium homeostasis interferes with aquaporin-2 (AQP-2) targeting to the apical membrane. Sustained PKA activation of PC2 and RyRs makes these channels leaky and leads to reduced intracellular calcium stores, further driving cAMP/PKA signaling. PKA activation also disrupts tubulogenesis, activates proproliferative signaling pathways, stimulates chloride and fluid secretion, and promotes STAT3-induced transcription of chemokines and cytokines. Vasopressin V2 and somatostatin (SST) stimulation of their respective receptors (V2R and SSTR) results in increased cAMP. Gs and Gi refer to guanosine nucleotide-binding proteins s and i, respectively. Yellow indicates proteins that are reduced in PKD; blue indicates proteins that are increased in PKD.
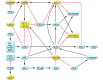
Figure 2. Network of pathways and transcription functions that regulate cell cycle progression, energy metabolism, and cell proliferation and death that are abnormal in PKD.
Upregulation of B-Raf/Mek/ERK, PI3K/AKT, and Wnt/β-catenin pathways and MYC and HIF transcription factors and downregulation of the LKB1/AMPK/TSC pathway, GSK3, and p53 promote aerobic glycolysis and cell cycle progression. Upregulation of MYC and downregulation of p53 exert proapoptotic and antiapoptotic effects, respectively. Downregulation of AMPK stimulates ion transport and fluid secretion. At multiple levels in this network, PKA activity stimulates proproliferative and inhibits antiproliferative signals. Yellow indicates proteins that are reduced in PKD; blue indicates proteins that are increased in PKD. OXPHOS, oxidative phosphorylation.
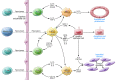
Figure 3. A model for the contribution of macrophages to PKD progression.
Activation of signaling pathways and transcription factors (e.g., STAT3, NF-κB) in cyst-lining cells stimulates the production and release of chemokines (e.g., MCP-1, osteopontin) attracting monocytes, promoting the polarization of invading monocytes and resident macrophages to a proinflammatory phenotype, and activating Th1 lymphocytes with further release of mediators and tissue damage. Opsonization of apoptotic cells by pentraxin-2 and secretion of IL-10 and TGF-β by immunosuppressive regulatory T cells promote the polarization of macrophages to a proproliferative phenotype, releasing antiinflammatory cytokines that induce cell proliferation. Incomplete epithelial healing, ongoing injury, and release of IL-4 and IL-13 by Th2 lymphocytes promote the polarization of macrophages to a profibrotic phenotype, releasing TGF-β and connective tissue growth factor (CTGF), which induces the differentiation of fibroblasts into collagen-secreting myofibroblasts.
Similar articles
- The heteromeric PC-1/PC-2 polycystin complex is activated by the PC-1 N-terminus.
Ha K, Nobuhara M, Wang Q, Walker RV, Qian F, Schartner C, Cao E, Delling M. Ha K, et al. Elife. 2020 Nov 9;9:e60684. doi: 10.7554/eLife.60684. Elife. 2020. PMID: 33164752 Free PMC article. - Molecular pathogenesis of autosomal dominant polycystic kidney disease.
Yoder BK, Mulroy S, Eustace H, Boucher C, Sandford R. Yoder BK, et al. Expert Rev Mol Med. 2006 Jan 17;8(2):1-22. doi: 10.1017/S1462399406010362. Expert Rev Mol Med. 2006. PMID: 16515728 Review. - Polycystin-1C terminus cleavage and its relation with polycystin-2, two proteins involved in polycystic kidney disease.
Bertuccio CA, Caplan MJ. Bertuccio CA, et al. Medicina (B Aires). 2013;73(2):155-62. Medicina (B Aires). 2013. PMID: 23570767 Review. - Molecular pathways and therapies in autosomal-dominant polycystic kidney disease.
Saigusa T, Bell PD. Saigusa T, et al. Physiology (Bethesda). 2015 May;30(3):195-207. doi: 10.1152/physiol.00032.2014. Physiology (Bethesda). 2015. PMID: 25933820 Free PMC article. Review. - Autosomal Dominant Polycystic Kidney Disease: A Path Forward.
Rangan GK, Lopez-Vargas P, Nankivell BJ, Tchan M, Tong A, Tunnicliffe DJ, Savige J. Rangan GK, et al. Semin Nephrol. 2015 Nov;35(6):524-37. doi: 10.1016/j.semnephrol.2015.10.002. Semin Nephrol. 2015. PMID: 26718155 Review.
Cited by
- Restoration of proximal tubule flow-activated transport prevents cyst growth in polycystic kidney disease.
Du Z, Tian X, Ma M, Somlo S, Weinstein AM, Wang T. Du Z, et al. JCI Insight. 2021 May 24;6(10):e146041. doi: 10.1172/jci.insight.146041. JCI Insight. 2021. PMID: 33886508 Free PMC article. - Virtual-tissue computer simulations define the roles of cell adhesion and proliferation in the onset of kidney cystic disease.
Belmonte JM, Clendenon SG, Oliveira GM, Swat MH, Greene EV, Jeyaraman S, Glazier JA, Bacallao RL. Belmonte JM, et al. Mol Biol Cell. 2016 Nov 7;27(22):3673-3685. doi: 10.1091/mbc.E16-01-0059. Epub 2016 May 18. Mol Biol Cell. 2016. PMID: 27193300 Free PMC article. - Prioritization of novel ADPKD drug candidates from disease-stage specific gene expression profiles.
Malas TB, Leonhard WN, Bange H, Granchi Z, Hettne KM, Van Westen GJP, Price LS, 't Hoen PAC, Peters DJM. Malas TB, et al. EBioMedicine. 2020 Jan;51:102585. doi: 10.1016/j.ebiom.2019.11.046. Epub 2019 Dec 24. EBioMedicine. 2020. PMID: 31879244 Free PMC article. - Proliferative signaling by ERBB proteins and RAF/MEK/ERK effectors in polycystic kidney disease.
Parker MI, Nikonova AS, Sun D, Golemis EA. Parker MI, et al. Cell Signal. 2020 Mar;67:109497. doi: 10.1016/j.cellsig.2019.109497. Epub 2019 Dec 9. Cell Signal. 2020. PMID: 31830556 Free PMC article. Review. - The lonidamine derivative H2-gamendazole reduces cyst formation in polycystic kidney disease.
Sundar SV, Zhou JX, Magenheimer BS, Reif GA, Wallace DP, Georg GI, Jakkaraj SR, Tash JS, Yu ASL, Li X, Calvet JP. Sundar SV, et al. Am J Physiol Renal Physiol. 2022 Oct 1;323(4):F492-F506. doi: 10.1152/ajprenal.00095.2022. Epub 2022 Aug 18. Am J Physiol Renal Physiol. 2022. PMID: 35979967 Free PMC article.
References
- Harris PC, Torres VE. Polycystic kidney disease, autosomal dominant. In: RA Pagon et al, eds. GeneReviews. Seattle, Washington, USA: University of Washington, Seattle; 2011. http://www.ncbi.nlm.nih.gov/books/NBK1246/. Updated 2011. Accessed May 5, 2014.
- Sweeney WE, Avner ED. Polycystic kidney disease, autosomal recessive. In: RA Pagon et al., eds. GeneReviews. Seattle, Washington, USA: University of Washington, Seattle; 1993–2014. http://www.ncbi.nlm.nih.gov/books/NBK1326/. Updated March 6, 2014. Accessed May 5, 2014.
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK090728/DK/NIDDK NIH HHS/United States
- DK058816/DK/NIDDK NIH HHS/United States
- DK044863/DK/NIDDK NIH HHS/United States
- DK090728/DK/NIDDK NIH HHS/United States
- R01 DK044863/DK/NIDDK NIH HHS/United States
- R01 DK059597/DK/NIDDK NIH HHS/United States
- R56 DK044863/DK/NIDDK NIH HHS/United States
- R01 DK058816/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases