Altered microglial response to Aβ plaques in APPPS1-21 mice heterozygous for TREM2 - PubMed (original) (raw)
Altered microglial response to Aβ plaques in APPPS1-21 mice heterozygous for TREM2
Jason D Ulrich et al. Mol Neurodegener. 2014.
Abstract
Background: Recent genome-wide association studies linked variants in TREM2 to a strong increase in the odds of developing Alzheimer's disease. The mechanism by which TREM2 influences the susceptibility to Alzheimer's disease is currently unknown. TREM2 is expressed by microglia and is thought to regulate phagocytic and inflammatory microglial responses to brain pathology. Given that a single allele of variant TREM2, likely resulting in a loss of function, conferred an increased risk of developing Alzheimer's disease, we tested whether loss of one functional trem2 allele would affect Aβ plaque deposition or the microglial response to Aβ pathology in APPPS1-21 mice.
Results: There was no significant difference in Aβ deposition in 3-month old or 7-month old APPPS1-21 mice expressing one or two copies of trem2. However, 3-month old mice with one copy of trem2 exhibited a marked decrease in the number and size of plaque-associated microglia. While there were no statistically significant differences in cytokine levels or markers of microglial activation in 3- or 7-month old animals, there were trends towards decreased expression of NOS2, C1qa, and IL1a in 3-month old TREM2+/- vs. TREM2+/+ mice.
Conclusions: Loss of a single copy of trem2 had no effect on Aβ pathology, but altered the morphological phenotype of plaque-associated microglia. These data suggest that TREM2 is important for the microglial response to Aβ deposition but that a 50% decrease inTREM2 expression does not affect Aβ plaque burden.
Figures
Figure 1
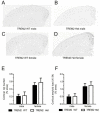
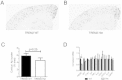
TREM2 heterozygosity does not affect Aβ plaque deposition in 3-month old APPPS1-21 mice. (A-D) Representative coronal brain sections from 3-month old from male TREM2 WT (A), male TREM2 Het (B), female TREM2 WT (C) and female TREM2 Het (D). Sections were immunostained with a biotinylated anti-Aβ antibody HJ3.4. (E) Quantification of the percentage of cortical area occupied by Aβ immunostaining. Two-way ANOVA analysis found a significant effect of gender (F1,39 = 13.63, p = 0.0007), but not genotype (F1,39 = 0.25, p = 0.62). (F) Quantification of the percentage of cortical area occupied by X-34 staining. Two-way ANOVA analysis found a significant effect of gender (F1,39 = 14.33, p = 0.0005), but not genotype (F1,39 = 0.08, p = 0.78; TREM2 WT (male, n = 12; female, n = 12) TREM2 Het (male, n = 9; female, n = 10)). Data are presented as mean ± SEM.
Figure 2
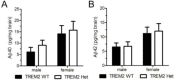
TREM2 heterozygosity does not affect PBS-insoluble Aβ levels in 3-month old APPPS1-21 mice. (A) Mean concentrations of PBS-insoluble Aβ40 in cortical tissue from TREM2 WT (male, n = 13; female, n = 12) and TREM2 Het (male, n = 8; female n = 10) mice were determined by ELISA. Two-way ANOVA analysis found a significant effect of gender (F1,38 = 5.49, p = 0.02), but not genotype (F1,38 = 0.55, p = 0.46). (B) Mean concentrations of PBS-insoluble Aβ42 in cortical tissue from TREM2 WT (male, n = 13; female, n = 12) and TREM2 Het (male, n = 8; female n = 10) mice were determined by ELISA. Two-way ANOVA analysis found a significant effect of gender (F1,38 = 5.96, p = 0.02), but not genotype (F1,38 = 0.07, p = 0.79). Data are presented as mean ± SEM.
Figure 3
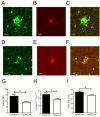
Decreased plaque-associated microglia in TREM2 Het mice. (A-F) Representative images of Alexa568-HJ3.4-stained plaque and GFP-expressing microglia from TREM2 WT (A-C) and TREM2 Het (D-F) mice. (G) The number of microglia per 100 μm2 within a 20 μm2 radius of an Aβ plaque in TREM2 WT mice (25.1 ± 2.25, n = 10) and TREM2 Het mice (18.3 ± 1.24, n = 9) was compared using a Mann–Whitney test (p = 0.03). (H) The mean soma size off plaque-associated microglia in TREM2 WT mice (45.0 ± 2.23 μm2, n = 10) and TREM2 Het mice (37.9 ± 1.57 μm2, n = 9) was compared using a Mann–Whitney test (p = 0.03). (I) The percent area covered by plaque-associated microglia in TREM2 WT mice (14.1 ± 1.4%, n = 10) and TREM2 Het mice (8.8 ± 0.71%, n = 9) was compared using a Mann–Whitney test (p = 0.01).
Figure 4
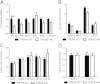
TREM2 heterozygosity does not significantly affect the inflammatory milieu in 3-month old APPPS1-21 mice. (A) Quantification of relative gene expression of microglial markers and NOS2 in TREM2 WT and TREM2 Het mice (n = 4-6 mice/genotype). For each mRNA analyzed TREM2 Het values were normalized and compared to TREM2 WT values using a t-test followed by a Benjamini-Hochberg p-value correction for multiple comparisons. (B-D) Levels of inflammatory cytokines in cortical tissue from TREM2 WT (n = 6) and TREM2 Het (n = 6) mice were compared using a t-test followed by Benjamini-Hochberg p-value correction for multiple comparisons. Cytokine levels are plotted on different axis for clarity of presentation. All data are presented as mean ± SEM, *corrected p < 0.05.
Figure 5
TREM2 hemizygosity does not significantly affect Aβ plaque burden or expression of M1/M2 microglial markers in 7-month old APPPS1-21 mice. (A and B) Representative coronal brain sections from 7-month old female TREM2 WT (A) and TREM2 Het (B) mice. Sections were immunostained with the biotinylated anti-Aβ antibody, HJ3.4. (C) Quantification of the cortical area occupied by Aβ immunostaining. TREM2 WT (5.85 ± 0.69%, n = 9) and TREM2 Het (4.60 ± 0.66%, n = 7) were statistically compared using a Mann Whitney test (p = 0.25). (D) Quantification of microglial mRNA expression in TREM2 WT and TREM2 Het mice. For each mRNA examined TREM2 Het were normalized and compared to TREM2 WT mice using a t-test followed by Benamini-Hochberg p-value correction for multiple comparisons. All data are presented as mean ± SEM (n = 3-7 mice per group), *p < 0.05.
Similar articles
- Trem2 Deletion Reduces Late-Stage Amyloid Plaque Accumulation, Elevates the Aβ42:Aβ40 Ratio, and Exacerbates Axonal Dystrophy and Dendritic Spine Loss in the PS2APP Alzheimer's Mouse Model.
Meilandt WJ, Ngu H, Gogineni A, Lalehzadeh G, Lee SH, Srinivasan K, Imperio J, Wu T, Weber M, Kruse AJ, Stark KL, Chan P, Kwong M, Modrusan Z, Friedman BA, Elstrott J, Foreman O, Easton A, Sheng M, Hansen DV. Meilandt WJ, et al. J Neurosci. 2020 Feb 26;40(9):1956-1974. doi: 10.1523/JNEUROSCI.1871-19.2019. Epub 2020 Jan 24. J Neurosci. 2020. PMID: 31980586 Free PMC article. - Effects of microglial depletion and TREM2 deficiency on Aβ plaque burden and neuritic plaque tau pathology in 5XFAD mice.
Delizannis AT, Nonneman A, Tsering W, De Bondt A, Van den Wyngaert I, Zhang B, Meymand E, Olufemi MF, Koivula P, Maimaiti S, Trojanowski JQ, Lee VM, Brunden KR. Delizannis AT, et al. Acta Neuropathol Commun. 2021 Sep 9;9(1):150. doi: 10.1186/s40478-021-01251-1. Acta Neuropathol Commun. 2021. PMID: 34503586 Free PMC article. - Intermittent hypoxia training enhances Aβ endocytosis by plaque associated microglia via VPS35-dependent TREM2 recycling in murine Alzheimer's disease.
Wang X, Xie Y, Fan X, Wu X, Wang D, Zhu L. Wang X, et al. Alzheimers Res Ther. 2024 Jun 3;16(1):121. doi: 10.1186/s13195-024-01489-6. Alzheimers Res Ther. 2024. PMID: 38831312 Free PMC article. - TREM2 Function in Alzheimer's Disease and Neurodegeneration.
Ulrich JD, Holtzman DM. Ulrich JD, et al. ACS Chem Neurosci. 2016 Apr 20;7(4):420-7. doi: 10.1021/acschemneuro.5b00313. Epub 2016 Feb 19. ACS Chem Neurosci. 2016. PMID: 26854967 Review. - TREM2 - a key player in microglial biology and Alzheimer disease.
Ulland TK, Colonna M. Ulland TK, et al. Nat Rev Neurol. 2018 Nov;14(11):667-675. doi: 10.1038/s41582-018-0072-1. Nat Rev Neurol. 2018. PMID: 30266932 Review.
Cited by
- Impact of TREM2R47H variant on tau pathology-induced gliosis and neurodegeneration.
Gratuze M, Leyns CE, Sauerbeck AD, St-Pierre MK, Xiong M, Kim N, Serrano JR, Tremblay MÈ, Kummer TT, Colonna M, Ulrich JD, Holtzman DM. Gratuze M, et al. J Clin Invest. 2020 Sep 1;130(9):4954-4968. doi: 10.1172/JCI138179. J Clin Invest. 2020. PMID: 32544086 Free PMC article. - Microglial INPP5D limits plaque formation and glial reactivity in the PSAPP mouse model of Alzheimer's disease.
Castranio EL, Hasel P, Haure-Mirande JV, Ramirez Jimenez AV, Hamilton BW, Kim RD, Glabe CG, Wang M, Zhang B, Gandy S, Liddelow SA, Ehrlich ME. Castranio EL, et al. Alzheimers Dement. 2023 Jun;19(6):2239-2252. doi: 10.1002/alz.12821. Epub 2022 Nov 30. Alzheimers Dement. 2023. PMID: 36448627 Free PMC article. - The involvement of microglia in Alzheimer's disease: a new dog in the fight.
Moore Z, Taylor JM, Crack PJ. Moore Z, et al. Br J Pharmacol. 2019 Sep;176(18):3533-3543. doi: 10.1111/bph.14546. Epub 2018 Dec 18. Br J Pharmacol. 2019. PMID: 30445661 Free PMC article. Review. - Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer's disease model.
Spangenberg E, Severson PL, Hohsfield LA, Crapser J, Zhang J, Burton EA, Zhang Y, Spevak W, Lin J, Phan NY, Habets G, Rymar A, Tsang G, Walters J, Nespi M, Singh P, Broome S, Ibrahim P, Zhang C, Bollag G, West BL, Green KN. Spangenberg E, et al. Nat Commun. 2019 Aug 21;10(1):3758. doi: 10.1038/s41467-019-11674-z. Nat Commun. 2019. PMID: 31434879 Free PMC article. - Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight.
Shi Y, Holtzman DM. Shi Y, et al. Nat Rev Immunol. 2018 Dec;18(12):759-772. doi: 10.1038/s41577-018-0051-1. Nat Rev Immunol. 2018. PMID: 30140051 Free PMC article. Review.
References
- Tanzi RE. In: The Biology of Alzheimer Disease. Selkoe DJ, Mandelkow E, Holtzman DM, editor. New York: Cold Spring Harbor Press; 2011. The Genetics of Alzheimer Disease; pp. 249–258.
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JSK, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J. TREM2 variants in Alzheimer’s disease. New Engl J Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. - DOI - PMC - PubMed
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K. Variant of TREM2 associated with the risk of Alzheimer’s disease. New Engl J Med. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous